Controlled spatial and conformational display of immobilised bone morphogenetic protein-2 and osteopontin signalling motifs regulates osteoblast adhesion and differentiation in vitro
- PMID: 20459712
- PMCID: PMC2880964
- DOI: 10.1186/1741-7007-8-57
Controlled spatial and conformational display of immobilised bone morphogenetic protein-2 and osteopontin signalling motifs regulates osteoblast adhesion and differentiation in vitro
Abstract
Background: The interfacial molecular mechanisms that regulate mammalian cell growth and differentiation have important implications for biotechnology (production of cells and cell products) and medicine (tissue engineering, prosthetic implants, cancer and developmental biology). We demonstrate here that engineered protein motifs can be robustly displayed to mammalian cells in vitro in a highly controlled manner using a soluble protein scaffold designed to self assemble on a gold surface.
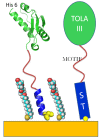
Results: A protein was engineered to contain a C-terminal cysteine that would allow chemisorption to gold, followed by 12 amino acids that form a water soluble coil that could switch to a hydrophobic helix in the presence of alkane thiols. Bioactive motifs from either bone morphogenetic protein-2 or osteopontin were added to this scaffold protein and when assembled on a gold surface assessed for their ability to influence cell function. Data demonstrate that osteoblast adhesion and short-term responsiveness to bone morphogenetic protein-2 is dependent on the surface density of a cell adhesive motif derived from osteopontin. Furthermore an immobilised cell interaction motif from bone morphogenetic protein supported bone formation in vitro over 28 days (in the complete absence of other osteogenic supplements). In addition, two-dimensional patterning of this ligand using a soft lithography approach resulted in the spatial control of osteogenesis.
Conclusion: These data describe an approach that allows the influence of immobilised protein ligands on cell behaviour to be dissected at the molecular level. This approach presents a durable surface that allows both short (hours or days) and long term (weeks) effects on cell activity to be assessed. This widely applicable approach can provide mechanistic insight into the contribution of immobilised ligands in the control of cell activity.
Figures


Comment in
-
Smart biomaterials - regulating cell behavior through signaling molecules.BMC Biol. 2010 May 19;8:59. doi: 10.1186/1741-7007-8-59. BMC Biol. 2010. PMID: 20529238 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

