Analysis of illegitimate genomic integration mediated by zinc-finger nucleases: implications for specificity of targeted gene correction
- PMID: 20459736
- PMCID: PMC2875229
- DOI: 10.1186/1471-2199-11-35
Analysis of illegitimate genomic integration mediated by zinc-finger nucleases: implications for specificity of targeted gene correction
Abstract
Background: Formation of site specific genomic double strand breaks (DSBs), induced by the expression of a pair of engineered zinc-finger nucleases (ZFNs), dramatically increases the rates of homologous recombination (HR) between a specific genomic target and a donor plasmid. However, for the safe use of ZFN induced HR in practical applications, possible adverse effects of the technology such as cytotoxicity and genotoxicity need to be well understood. In this work, off-target activity of a pair of ZFNs has been examined by measuring the ratio between HR and illegitimate genomic integration in cells that are growing exponentially, and in cells that have been arrested in the G2/M phase.
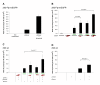
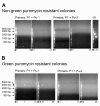
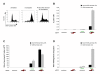
Results: A reporter cell line that contained consensus ZFN binding sites in an enhanced green fluorescent protein (EGFP) reporter gene was used to measure ratios between HR and non-homologous integration of a plasmid template. Both in human cells (HEK 293) containing the consensus ZFN binding sites and in cells lacking the ZFN binding sites, a 3.5 fold increase in the level of illegitimate integration was observed upon ZFN expression. Since the reporter gene containing the consensus ZFN target sites was found to be intact in cells where illegitimate integration had occurred, increased rates of illegitimate integration most likely resulted from the formation of off-target genomic DSBs. Additionally, in a fraction of the ZFN treated cells the co-occurrence of both specific HR and illegitimate integration was observed. As a mean to minimize unspecific effects, cell cycle manipulation of the target cells by induction of a transient G2/M cell cycle arrest was shown to stimulate the activity of HR while having little effect on the levels of illegitimate integration, thus resulting in a nearly eight fold increase in the ratio between the two processes.
Conclusions: The demonstration that ZFN expression, in addition to stimulating specific gene targeting by HR, leads to increased rates of illegitimate integration emphasizes the importance of careful characterization of ZFN treated cells. In order to reduce off-target events, reversible cell cycle arrest of the target cells in the G2/M phase is an efficient way for increasing the ratio between specific HR and illegitimate integration.
Figures
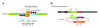

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

