Subchronic exposure to phytoestrogens alone and in combination with diethylstilbestrol - pituitary tumor induction in Fischer 344 rats
- PMID: 20459739
- PMCID: PMC2881934
- DOI: 10.1186/1743-7075-7-40
Subchronic exposure to phytoestrogens alone and in combination with diethylstilbestrol - pituitary tumor induction in Fischer 344 rats
Abstract
Background: Subchronic administration of the potent pharmaceutical estrogen diethylstilbestrol (DES) to female Fischer 344 (F344) rats induces growth of large, hemorrhagic pituitaries that progress to tumors. Phytoestrogens (dietary plant estrogens) are hypothesized to be potential tumor inhibitors in tissues prone to estrogen-induced cancers, and have been suggested as "safer" estrogen replacements. However, it is unknown if they might themselves establish or exacerbate the growth of estrogen-responsive cancers, such as in pituitary.
Methods: We implanted rats with silastic capsules containing 5 mg of four different phytoestrogens - either coumestrol, daidzein, genistein, or trans-resveratrol, in the presence or absence of DES. We examined pituitary and other organ weights, blood levels of prolactin (PRL) and growth hormone (GH), body weights, and pituitary tissue histology.
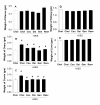
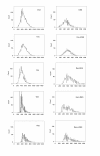
Results: Blood level measurements of the administered phytoestrogens confirmed successful exposure of the animals to high levels of these compounds. By themselves, no phytoestrogen increased pituitary weights or serum PRL levels after 10 weeks of treatment. DES, genistein, and resveratrol increased GH levels during this time. Phytoestrogens neither changed any wet organ weight (uterus, ovary, cervix, liver, and kidney) after 10 weeks of treatment, nor reversed the adverse effects of DES on pituitaries, GH and PRL levels, or body weight gain after 8 weeks of co-treatment. However, they did reverse the DES-induced weight increase on the ovary and cervix. Morphometric examination of pituitaries revealed that treatment with DES, either alone or in combination with phytoestrogens, caused gross structural changes that included decreases in tissue cell density, increases in vascularity, and multiple hemorrhagic areas. DES, especially in combination with phytoestrogens, caused the development of larger and more heterogeneous nuclear sizes in pituitary.
Conclusions: High levels of phytoestrogens by themselves did not cause pituitary precancerous growth or change weights of other estrogen-sensitive organs, though when combined with DES, they counteracted the growth effects of DES on reproductive organs. In the pituitary, phytoestrogens did not reverse the effects of DES, but they did increase the sizes and size heterogeneity of nuclei. Therefore, phytoestrogens may oppose some but not all estrogen-responsive tissue abnormalities caused by DES overstimulation, and appear to exacerbate DES-induced nuclear changes.
Figures





Similar articles
-
Regulation of GH and TSH release from hyperplastic and ectopic pituitaries: effects of dopamine in vitro.Life Sci. 1989;45(3):199-206. doi: 10.1016/0024-3205(89)90251-8. Life Sci. 1989. PMID: 2761337
-
Glandular kallikrein in estrogen-induced pituitary tumors: time course of induction and correlation with prolactin.Cancer Res. 1988 Aug 1;48(15):4158-62. Cancer Res. 1988. PMID: 3390808
-
Estrogen induction of prolactin-producing pituitary tumors in the Fischer 344 rat: modulation by dietary-energy but not protein consumption.Mol Carcinog. 1998 Oct;23(2):96-105. Mol Carcinog. 1998. PMID: 9808163
-
Phytoestrogens: potential endocrine disruptors in males.Toxicol Ind Health. 1998 Jan-Apr;14(1-2):223-37. doi: 10.1177/074823379801400114. Toxicol Ind Health. 1998. PMID: 9460177 Review.
-
The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens.Biomedicines. 2024 Jul 23;12(8):1636. doi: 10.3390/biomedicines12081636. Biomedicines. 2024. PMID: 39200101 Free PMC article. Review.
Cited by
-
Chronic estradiol exposure induces oxidative stress in the hypothalamus to decrease hypothalamic dopamine and cause hyperprolactinemia.Am J Physiol Regul Integr Comp Physiol. 2011 Mar;300(3):R693-9. doi: 10.1152/ajpregu.00481.2010. Epub 2010 Dec 22. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21178126 Free PMC article.
-
Phytoestrogens and Their Health Effect.Open Access Maced J Med Sci. 2019 Feb 14;7(3):495-499. doi: 10.3889/oamjms.2019.044. eCollection 2019 Feb 15. Open Access Maced J Med Sci. 2019. PMID: 30834024 Free PMC article. Review.
-
Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells.Environ Health Perspect. 2011 Jan;119(1):104-12. doi: 10.1289/ehp.1002512. Epub 2010 Sep 22. Environ Health Perspect. 2011. PMID: 20870566 Free PMC article.
-
Combinations of physiologic estrogens with xenoestrogens alter calcium and kinase responses, prolactin release, and membrane estrogen receptor trafficking in rat pituitary cells.Environ Health. 2010 Oct 15;9:61. doi: 10.1186/1476-069X-9-61. Environ Health. 2010. PMID: 20950447 Free PMC article.
-
Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells.Environ Health. 2013 Mar 26;12:26. doi: 10.1186/1476-069X-12-26. Environ Health. 2013. PMID: 23530988 Free PMC article.
References
-
- Sarkar DK, Hentges ST, De A, Reddy RH. Hormonal control of pituitary prolactin-secreting tumors. Front Biosci. 1998;3:d934–d943. - PubMed
-
- Cracchiolo D, Swick JW, McKiernan L, Sloan E, Raina S, Sloan C, Wendell DL. Estrogen-dependent growth of a rat pituitary tumor involves, but does not require, a high level of vascular endothelial growth factor. Exp Biol Med (Maywood) 2002;227:492–499. - PubMed
LinkOut - more resources
Full Text Sources

