Diametrically opposed effects of hypoxia and oxidative stress on two viral transactivators
- PMID: 20459757
- PMCID: PMC2874542
- DOI: 10.1186/1743-422X-7-93
Diametrically opposed effects of hypoxia and oxidative stress on two viral transactivators
Abstract
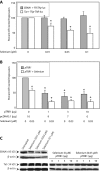
Background: Many pathogens exist in multiple physiological niches within the host. Differences between aerobic and anaerobic conditions are known to alter the expression of bacterial virulence factors, typically through the conditional activity of transactivators that modulate their expression. More recently, changes in physiological niches have been shown to affect the expression of viral genes. For many viruses, differences in oxygen tension between hypoxia and normoxia alter gene expression or function. Oxygen tension also affects many mammalian transactivators including AP-1, NFkB, and p53 by affecting the reduced state of critical cysteines in these proteins. We have recently determined that an essential cys-x-x-cys motif in the EBNA1 transactivator of Epstein-Barr virus is redox-regulated, such that transactivation is favoured under reducing conditions. The crucial Tat transactivator of human immunodeficiency virus (HIV) has an essential cysteine-rich region, and is also regulated by redox. Contrary to EBNA1, it is reported that Tat's activity is increased by oxidative stress. Here we have compared the effects of hypoxia, oxidative stress, and cellular redox modulators on EBNA1 and Tat.
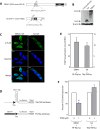
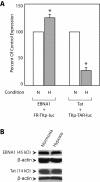
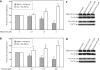
Results: Our results indicate that unlike EBNA1, Tat is less active during hypoxia. Agents that generate hydroxyl and superoxide radicals reduce EBNA1's activity but increase transactivation by Tat. The cellular redox modulator, APE1/Ref-1, increases EBNA1's activity, without any effect on Tat. Conversely, thioredoxin reductase 1 (TRR1) reduces Tat's function without any effect on EBNA1.
Conclusions: We conclude that oxygen partial pressure and oxidative stress affects the functions of EBNA1 and Tat in a dramatically opposed fashion. Tat is more active during oxidative stress, whereas EBNA1's activity is compromised under these conditions. The two proteins respond to differing cellular redox modulators, suggesting that the oxidized cysteine adduct is a disulfide bond(s) in Tat, but sulfenic acid in EBNA1. The effect of oxygen partial pressure on transactivator function suggests that changes in redox may underlie differences in virus-infected cells dependent upon the physiological niches they traffic to.
Figures
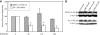
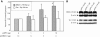
Similar articles
-
Zinc coordination is required for and regulates transcription activation by Epstein-Barr nuclear antigen 1.PLoS Pathog. 2009 Jun;5(6):e1000469. doi: 10.1371/journal.ppat.1000469. Epub 2009 Jun 12. PLoS Pathog. 2009. PMID: 19521517 Free PMC article.
-
Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation.Mol Cancer. 2010 Jan 5;9:1. doi: 10.1186/1476-4598-9-1. Mol Cancer. 2010. PMID: 20051109 Free PMC article.
-
Optimal transactivation by Epstein-Barr nuclear antigen 1 requires the UR1 and ATH1 domains.J Virol. 2009 May;83(9):4227-35. doi: 10.1128/JVI.02578-08. Epub 2009 Feb 25. J Virol. 2009. PMID: 19244333 Free PMC article.
-
Potential cellular functions of Epstein-Barr Nuclear Antigen 1 (EBNA1) of Epstein-Barr Virus.Viruses. 2013 Jan 16;5(1):226-40. doi: 10.3390/v5010226. Viruses. 2013. PMID: 23325328 Free PMC article. Review.
-
Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival.Viruses. 2012 Sep;4(9):1537-1547. doi: 10.3390/v4091537. Epub 2012 Sep 13. Viruses. 2012. PMID: 23170171 Free PMC article. Review.
Cited by
-
Antiviral activity of diallyl trisulfide against H9N2 avian influenza virus infection in vitro and in vivo.Virol J. 2021 Aug 19;18(1):171. doi: 10.1186/s12985-021-01641-w. Virol J. 2021. PMID: 34412671 Free PMC article.
-
Ammonia generation by tryptophan synthase drives a key genetic difference between genital and ocular Chlamydia trachomatis isolates.Proc Natl Acad Sci U S A. 2019 Jun 18;116(25):12468-12477. doi: 10.1073/pnas.1821652116. Epub 2019 May 16. Proc Natl Acad Sci U S A. 2019. PMID: 31097582 Free PMC article.
-
Dinosolve: a protein disulfide bonding prediction server using context-based features to enhance prediction accuracy.BMC Bioinformatics. 2013;14 Suppl 13(Suppl 13):S9. doi: 10.1186/1471-2105-14-S13-S9. Epub 2013 Oct 1. BMC Bioinformatics. 2013. PMID: 24267383 Free PMC article.
-
Hypoxia induces the gene expression and extracellular transmission of persistent lymphocytic choriomeningitis virus.J Virol. 2011 Dec;85(24):13069-76. doi: 10.1128/JVI.00829-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957293 Free PMC article.
-
Oxygen Sensing and Viral Replication: Implications for Tropism and Pathogenesis.Viruses. 2020 Oct 25;12(11):1213. doi: 10.3390/v12111213. Viruses. 2020. PMID: 33113858 Free PMC article. Review.
References
-
- Krieger JA, Landsiedel JC, Lawrence DA. Differential in vitro effects of physiological and atmospheric oxygen tension on normal human peripheral blood mononuclear cell proliferation, cytokine and immunoglobulin production. Int J Immunopharmacol. 1996;18:545–552. doi: 10.1016/S0192-0561(96)00057-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

