T cells, adhesion molecules and modulation of apoptosis in visceral leishmaniasis glomerulonephritis
- PMID: 20459816
- PMCID: PMC2880314
- DOI: 10.1186/1471-2334-10-112
T cells, adhesion molecules and modulation of apoptosis in visceral leishmaniasis glomerulonephritis
Abstract
Background: Immune complex deposition is the accepted mechanism of pathogenesis of VL glomerulopathy however other immune elements may participate. Further in the present study, no difference was seen between immunoglobulin and C3b deposit intensity in glomeruli between infected and non-infected dogs thus T cells, adhesion molecules and parameters of proliferation and apoptosis were analysed in dogs with naturally acquired VL from an endemic area. The dog is the most important domestic reservoir of the protozoa Leishmania (L.) chagasi that causes visceral leishmaniasis (VL). The similarity of VL manifestation in humans and dogs renders the study of canine VL nephropathy of interest with regard to human pathology.
Methods: From 55 dogs with VL and 8 control non-infected dogs from an endemic area, kidney samples were analyzed by immunohistochemistry for immunoglobulin and C3b deposits, staining for CD4+ and CD8+ T cells, ICAM-1, P-selectin and quantified using morphometry. Besides proliferation marker Ki-67, apoptosis markers M30 and TUNEL staining, and related cytokines TNF-alpha, IL-1alpha were searched and quantified.
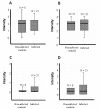
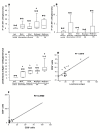
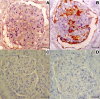
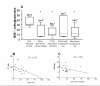
Results: We observed similar IgG, IgM and IgA and C3b deposit intensity in dogs with VL and non-infected control dogs. However we detected the Leishmania antigen in cells in glomeruli in 54, CD4+ T cells in the glomeruli of 44, and CD8+ T cells in 17 of a total of 55 dogs with VL. Leishmania antigen was absent and T cells were absent/scarse in eight non-infected control dogs. CD 4+ T cells predominate in proliferative patterns of glomerulonephritis, however the presence of CD4+ and CD8+ T cells were not different in intensity in different patterns of glomerulonephritis. The expression of ICAM-1 and P-selectin was significantly greater in the glomeruli of infected dogs than in control dogs. In all patterns of glomerulonephritis the expression of ICAM-1 ranged from minimum to moderately severe and P-selectin from absent to severe. In the control animals the expression of these molecules ranged from absent to medium intensity. It was not observed any correlation between severity of the disease and these markers. There was a correlation between the number of Leishmania antigen positive cells and CD4+ T cells, and between the number of CD4+ T cells and CD8+ T cells. In dogs presenting different histopathological patterns of glomerulonephritis, parameters of proliferation and apoptosis were studied. Ki-67, a proliferative marker, was not detected locally, but fewer apoptotic cells and lower TNF-alpha expression were seen in infected animals than in non-infected controls.
Conclusion: Immunopathogenic mechanisms of VL glomerulonephritis are complex and data in the present study suggest no clear participation of immunoglobulin and C3b deposits in these dogs but the possible migration of CD4+ T cells into the glomeruli, participation of adhesion molecules, and diminished apoptosis of cells contributing to determine the proliferative pattern of glomerulonephritis in VL.
Figures



Similar articles
-
CD4(+) T cells participate in the nephropathy of canine visceral leishmaniasis.Braz J Med Biol Res. 2000 Dec;33(12):1455-8. doi: 10.1590/s0100-879x2000001200009. Braz J Med Biol Res. 2000. PMID: 11105098
-
IL-10 receptor blockade controls the in vitro infectivity of Leishmania infantum and promotes a Th1 activation in PBMC of dogs with visceral leishmaniasis.Mol Immunol. 2021 Sep;137:20-27. doi: 10.1016/j.molimm.2021.06.014. Epub 2021 Jun 26. Mol Immunol. 2021. PMID: 34182228
-
Recovery of antigen-specific T cell responses from dogs infected with Leishmania (L.) infantum by use of vaccine associated TLR-agonist adjuvant.Vaccine. 2016 Oct 17;34(44):5225-5234. doi: 10.1016/j.vaccine.2016.09.016. Epub 2016 Sep 21. Vaccine. 2016. PMID: 27665354 Free PMC article.
-
Systemic and compartmentalized immune response in canine visceral leishmaniasis.Vet Immunol Immunopathol. 2009 Mar 15;128(1-3):87-95. doi: 10.1016/j.vetimm.2008.10.307. Epub 2008 Oct 17. Vet Immunol Immunopathol. 2009. PMID: 19054576 Review.
-
Cutaneous immune mechanisms in canine leishmaniosis due to Leishmania infantum.Vet Immunol Immunopathol. 2015 Feb 15;163(3-4):94-102. doi: 10.1016/j.vetimm.2014.11.011. Epub 2014 Nov 20. Vet Immunol Immunopathol. 2015. PMID: 25555497 Review.
Cited by
-
Immunohistochemical study of renal fibropoiesis associated with dogs naturally and experimentally infected with two different strains of Leishmania (L.) infantum.Int J Exp Pathol. 2019 Aug;100(4):222-233. doi: 10.1111/iep.12321. Epub 2019 Nov 6. Int J Exp Pathol. 2019. PMID: 31696580 Free PMC article.
-
Kidney involvement in leishmaniasis--a review.Braz J Infect Dis. 2014 Jul-Aug;18(4):434-40. doi: 10.1016/j.bjid.2013.11.013. Epub 2014 Mar 29. Braz J Infect Dis. 2014. PMID: 24690431 Free PMC article. Review.
-
A compared histopathological study on kidneys and eye bulbs in distinct clinical presentations of canine leishmaniasis by Leishmania infantum.Vet Res Commun. 2024 Aug;48(4):2243-2261. doi: 10.1007/s11259-024-10379-z. Epub 2024 May 8. Vet Res Commun. 2024. PMID: 38717733
-
Novel kidney injury biomarkers in tropical infections: a review of the literature.Rev Inst Med Trop Sao Paulo. 2020 Feb 14;62:e14. doi: 10.1590/S1678-9946202062014. eCollection 2020. Rev Inst Med Trop Sao Paulo. 2020. PMID: 32074217 Free PMC article. Review.
-
Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia.Acta Pharmacol Sin. 2010 Oct;31(10):1395-400. doi: 10.1038/aps.2010.167. Epub 2010 Sep 27. Acta Pharmacol Sin. 2010. PMID: 20871618 Free PMC article.
References
-
- Lindoso JAL, Goto H. In: Tratado de Clínica Médica. 1. Editora Roca L, editor. São Paulo, SP.: Lopes, A. C. & Amato Neto, V; 2006. Leishmaniose Visceral; pp. 4121–4125.
-
- de Brito T, Hoshino-Shimizu S, Neto VA, Duarte IS, Penna DO. Glomerular involvement in human kala-azar. A light, immunofluorescent, and electron microscopic study based on kidney biopsies. Am J Trop MedHyg. 1975;24:9–18. - PubMed
-
- Weisinger JR, Pinto A, Velazquez GA, Bronstein I, Dessene JJ, Duque JF, Montenegro J, Tapanes F, de Rousse AR. Clinical and histological kidney involvement in human kala-azar. Am J Trop Med Hyg. 1978;27:357–359. - PubMed
-
- Mancianti F, Poli A, Bionda A. Analysis of renal immune-deposits in canine leishmaniasis. Preliminary results. Parassitologia. 1989;31:213–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

