Experimental depletion of CD8+ cells in acutely SIVagm-infected African Green Monkeys results in increased viral replication
- PMID: 20459829
- PMCID: PMC2879233
- DOI: 10.1186/1742-4690-7-42
Experimental depletion of CD8+ cells in acutely SIVagm-infected African Green Monkeys results in increased viral replication
Abstract
Background: In vivo CD8+ cell depletions in pathogenic SIV infections identified a key role for cellular immunity in controlling viral load (VL) and disease progression. However, similar studies gave discordant results in chronically-infected SMs, leading some authors to propose that in natural hosts, SIV replication is independent of cellular immunity. To assess the role of cellular immune responses in the control of SIV replication in natural hosts, we investigated the impact of CD8+ cell depletion during acute SIV infection in AGMs.
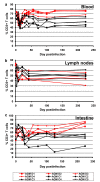
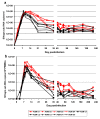
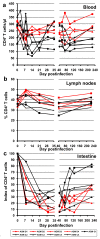
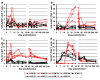
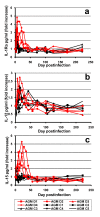
Results: Nine AGMs were infected with SIVagm.sab and were followed up to day 225 p.i. Four were intravenously infused with the cM-T807 antibody on days 0 (50 mg/kg), 6, and 13 (10 mg/kg, respectively) post infection (p.i.). CD8+ cells were depleted for up to 28 days p.i. in peripheral blood and LNs in all treated AGMs. Partial CD8+ T cell depletion occurred in the intestine. SIVagm VLs peaked at similar levels in both groups (107-108 RNA copies/ml). However, while VLs were controlled in undepleted AGMs, reaching set-point levels (104-105 RNA copies/ml) by day 28 p.i., high VLs (>106 RNA copies/ml) were maintained by day 21 p.i. in CD8-depleted AGMs. By day 42 p.i., VLs were comparable between the two groups. The levels of immune activation and proliferation remained elevated up to day 72 p.i. in CD8-depleted AGMs and returned to preinfection levels in controls by day 28 p.i. None of the CD8-depleted animals progressed to AIDS.
Conclusion: CD8+ cells are responsible for a partial control of postacute viral replication in SIVagm.sab-infected AGMs. In contrast to macaques, the SIVagm-infected AGMs are able to control viral replication after recovery of the CD8+ T cells and avoid disease progression.
Figures

Similar articles
-
Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host.J Virol. 2009 Oct;83(20):10347-57. doi: 10.1128/JVI.00880-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656874 Free PMC article.
-
Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts.J Virol. 2006 May;80(10):4858-67. doi: 10.1128/JVI.80.10.4858-4867.2006. J Virol. 2006. PMID: 16641277 Free PMC article.
-
High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys.J Virol. 2000 Aug;74(16):7538-47. doi: 10.1128/jvi.74.16.7538-7547.2000. J Virol. 2000. PMID: 10906207 Free PMC article.
-
SIVagm: genetic and biological features associated with replication.Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
-
What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS?AIDS Rev. 2004 Jan-Mar;6(1):40-53. AIDS Rev. 2004. PMID: 15168740 Review.
Cited by
-
Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys.Nat Med. 2017 Nov;23(11):1277-1286. doi: 10.1038/nm.4421. Epub 2017 Oct 16. Nat Med. 2017. PMID: 29035370 Free PMC article.
-
SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations.PLoS Pathog. 2013 Jan;9(1):e1003011. doi: 10.1371/journal.ppat.1003011. Epub 2013 Jan 17. PLoS Pathog. 2013. PMID: 23349627 Free PMC article.
-
Envelope-specific B-cell populations in African green monkeys chronically infected with simian immunodeficiency virus.Nat Commun. 2016 Jul 6;7:12131. doi: 10.1038/ncomms12131. Nat Commun. 2016. PMID: 27381634 Free PMC article.
-
The well-tempered SIV infection: Pathogenesis of SIV infection in natural hosts in the wild, with emphasis on virus transmission and early events post-infection that may contribute to protection from disease progression.Infect Genet Evol. 2016 Dec;46:308-323. doi: 10.1016/j.meegid.2016.07.006. Epub 2016 Jul 6. Infect Genet Evol. 2016. PMID: 27394696 Free PMC article.
-
Immunodeficiency lentiviral infections in natural and non-natural hosts.Blood. 2011 Jul 28;118(4):847-54. doi: 10.1182/blood-2010-12-325936. Epub 2011 Apr 19. Blood. 2011. PMID: 21505193 Free PMC article. Review.
References
-
- Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, Kaur A, Lackner AA, Veazey RS, Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. - DOI - PMC - PubMed
-
- Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch VM, Apetrei C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol. 2006;35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. - DOI - PubMed
-
- Apetrei C, Gautam R, Sumpter B, Carter AC, Gaufin T, Staprans SI, Else J, Barnes M, Cao R Jr, Garg S, Milush JM, Sodora DL, Pandrea I, Silvestri G. Virus-subtype specific features of natural SIVsmm infection in sooty mangabeys. J Virol. 2007;81:7913–7923. doi: 10.1128/JVI.00281-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

