Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice
- PMID: 20459836
- PMCID: PMC2875211
- DOI: 10.1186/1710-1492-6-7
Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice
Abstract
Background: Multiple studies have suggested that prenatal exposure to either allergens or air pollution may increase the risk for the development of allergic immune responses in young offspring. However, the effects of prenatal environmental exposures on adult offspring have not been well-studied. We hypothesized that combined prenatal exposure to Aspergillus fumigatus (A. fumigatus) allergen and diesel exhaust particles will be associated with altered IgE production, airway inflammation, airway hyperreactivity (AHR), and airway remodeling of adult offspring.
Methods: Following sensitization via the airway route to A. fumigatus and mating, pregnant BALB/c mice were exposed to additional A. fumigatus and/or diesel exhaust particles. At age 9-10 weeks, their offspring were sensitized and challenged with A. fumigatus.
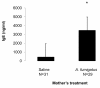
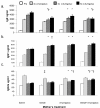
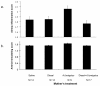
Results: We found that adult offspring from mice that were exposed to A. fumigatus or diesel exhaust particles during pregnancy experienced decreases in IgE production. Adult offspring of mice that were exposed to both A. fumigatus and diesel exhaust particles during pregnancy experienced decreases in airway eosinophilia.
Conclusion: These results suggest that, in this model, allergen and/or diesel administration during pregnancy may be associated with protection from developing systemic and airway allergic immune responses in the adult offspring.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

