Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors
- PMID: 20459837
- PMCID: PMC2881878
- DOI: 10.1186/1471-230X-10-44
Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors
Abstract
Background: Gene transfer to the gastrointestinal (GI) mucosa is a therapeutic strategy which could prove particularly advantageous for treatment of various hereditary and acquired intestinal disorders, including inflammatory bowel disease (IBD), GI infections, and cancer.
Methods: We evaluated vesicular stomatitis virus glycoprotein envelope (VSV-G)-pseudotyped lentiviral vectors (LV) for efficacy of gene transfer to both murine rectosigmoid colon in vivo and human colon explants ex vivo. LV encoding beta-galactosidase (LV-beta-Gal) or firefly-luciferase (LV-fLuc) reporter genes were administered by intrarectal instillation in mice, or applied topically for ex vivo transduction of human colorectal explant tissues from normal individuals. Macroscopic and histological evaluations were performed to assess any tissue damage or inflammation. Transduction efficiency and systemic biodistribution were evaluated by real-time quantitative PCR. LV-fLuc expression was evaluated by ex vivo bioluminescence imaging. LV-beta-Gal expression and identity of transduced cell types were examined by histochemical and immunofluorescence staining.
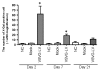
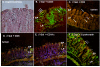
Results: Imaging studies showed positive fLuc signals in murine distal colon; beta-Gal-positive cells were found in both murine and human intestinal tissue. In the murine model, beta-Gal-positive epithelial and lamina propria cells were found to express cytokeratin, CD45, and CD4. LV-transduced beta-Gal-positive cells were also seen in human colorectal explants, consisting mainly of CD45, CD4, and CD11c-positive cells confined to the LP.
Conclusions: We have demonstrated the feasibility of LV-mediated gene transfer into colonic mucosa. We also identified differential patterns of mucosal gene transfer dependent on whether murine or human tissue was used. Within the limitations of the study, the LV did not appear to induce mucosal damage and were not distributed beyond the distal colon.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

