Protocol for the MoleMate UK Trial: a randomised controlled trial of the MoleMate system in the management of pigmented skin lesions in primary care [ISRCTN 79932379]
- PMID: 20459846
- PMCID: PMC2881908
- DOI: 10.1186/1471-2296-11-36
Protocol for the MoleMate UK Trial: a randomised controlled trial of the MoleMate system in the management of pigmented skin lesions in primary care [ISRCTN 79932379]
Abstract
Background: Suspicious pigmented lesions are a common presenting problem in general practice consultations; while the majority are benign a small minority are melanomas. Differentiating melanomas from other pigmented lesions in primary care is challenging: currently, 95% of all lesions referred to a UK specialist are benign. The MoleMate system is a new diagnostic aid, incorporating a hand-held SIAscopy scanner with a primary care diagnostic algorithm. This trial tests the hypothesis that adding the MoleMate system to current best primary care practice will increase the proportion of appropriate referrals of suspicious pigmented lesions to secondary care compared with current best practice alone.
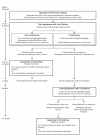
Methods/design: The MoleMate UK Trial is a primary care based multi-centre randomised controlled trial, with randomisation at patient level using a validated block randomisation method for two age groups (45 years and under; 46 years and over). We aim to recruit adult patients seen in general practice with a pigmented skin lesion that cannot immediately be diagnosed as benign and the patient reassured. The trial has a 'two parallel groups' design, comparing 'best practice' with 'best practice' plus the MoleMate system in the intervention group. The primary outcome is the positive predictive value (PPV) of referral defined as the proportion of referred lesions seen by secondary care experts that are considered 'clinically significant' (i.e. biopsied or monitored). Secondary outcomes include: the sensitivity, specificity and negative predictive value (NPV) of the decision not to refer; clinical outcomes (melanoma thickness, 5 year melanoma incidence and mortality); clinician outcomes (Index of Suspicion, confidence, learning effects); patient outcomes (satisfaction, general and cancer-specific worry), and cost-utility.
Discussion: The MoleMate UK Trial tests a new technology designed to improve the management of suspicious pigmented lesions in primary care. If effective, the MoleMate system could reduce the burden on skin cancer clinics of patients with benign pigmented skin lesions, and improve patient care in general practice.
References
-
- CRUK. The National Awareness and Early Diagnosis Inititative (NAEDI) 2008.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources