Recombinant lambda-phage nanobioparticles for tumor therapy in mice models
- PMID: 20459865
- PMCID: PMC2890663
- DOI: 10.1186/1479-0556-8-3
Recombinant lambda-phage nanobioparticles for tumor therapy in mice models
Abstract
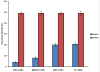
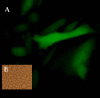
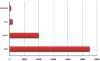
Lambda phages have considerable potential as gene delivery vehicles due to their genetic tractability, low cost, safety and physical characteristics in comparison to other nanocarriers and gene porters. Little is known concerning lambda phage-mediated gene transfer and expression in mammalian hosts. We therefore performed experiments to evaluate lambda-ZAP bacteriophage-mediated gene transfer and expression in vitro. For this purpose, we constructed recombinant lambda-phage nanobioparticles containing a mammalian expression cassette encoding enhanced green fluorescent protein (EGFP) and E7 gene of human papillomavirus type 16 (lambda-HPV-16 E7) using Lambda ZAP- CMV XR vector. Four cell lines (COS-7, CHO, TC-1 and HEK-239) were transduced with the nanobioparticles. We also characterized the therapeutic anti-tumor effects of the recombinant lambda-HPV-16 E7 phage in C57BL/6 tumor mice model as a cancer vaccine. Obtained results showed that delivery and expression of these genes in fibroblastic cells (COS-7 and CHO) are more efficient than epithelial cells (TC-1 and HEK-239) using these nanobioparticles. Despite the same phage M.O.I entry, the internalizing titers of COS-7 and CHO cells were more than TC-1 and HEK-293 cells, respectively. Mice vaccinated with lambda-HPV-16 E7 are able to generate potent therapeutic antitumor effects against challenge with E7- expressing tumor cell line, TC-1 compared to group treated with the wild phage. The results demonstrated that the recombinant lambda-phages, due to their capabilities in transducing mammalian cells, can also be considered in design and construction of novel and safe phage-based nanomedicines.
Figures



Similar articles
-
Recombinant λ bacteriophage displaying nanobody towards third domain of HER-2 epitope inhibits proliferation of breast carcinoma SKBR-3 cell line.Arch Immunol Ther Exp (Warsz). 2013 Feb;61(1):75-83. doi: 10.1007/s00005-012-0206-x. Epub 2012 Dec 7. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23224340
-
λ Phage nanobioparticle expressing apoptin efficiently suppress human breast carcinoma tumor growth in vivo.PLoS One. 2013 Nov 22;8(11):e79907. doi: 10.1371/journal.pone.0079907. eCollection 2013. PLoS One. 2013. PMID: 24278212 Free PMC article.
-
In vivo gene delivery and expression by bacteriophage lambda vectors.J Appl Microbiol. 2007 May;102(5):1337-49. doi: 10.1111/j.1365-2672.2006.03182.x. J Appl Microbiol. 2007. PMID: 17448169 Free PMC article.
-
Recombinant DNA vaccines protect against tumors that are resistant to recombinant vaccinia vaccines containing the same gene.Gene Ther. 2001 Jan;8(2):128-38. doi: 10.1038/sj.gt.3301370. Gene Ther. 2001. PMID: 11313782
-
Genetic immunisation against hepatitis B using whole bacteriophage lambda particles.Vaccine. 2004 Apr 16;22(13-14):1666-71. doi: 10.1016/j.vaccine.2003.10.047. Vaccine. 2004. PMID: 15068849
Cited by
-
Engineering T7 bacteriophage as a potential DNA vaccine targeting delivery vector.Virol J. 2018 Mar 20;15(1):49. doi: 10.1186/s12985-018-0955-1. Virol J. 2018. PMID: 29558962 Free PMC article.
-
Phage based vaccine: A novel strategy in prevention and treatment.Heliyon. 2023 Sep 15;9(9):e19925. doi: 10.1016/j.heliyon.2023.e19925. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809683 Free PMC article. Review.
-
Phage engineering and the evolutionary arms race.Curr Opin Biotechnol. 2021 Apr;68:23-29. doi: 10.1016/j.copbio.2020.09.009. Epub 2020 Oct 23. Curr Opin Biotechnol. 2021. PMID: 33113495 Free PMC article. Review.
-
Cysteine/Histidine-Dependent Amidohydrolase/Peptidase (CHAP)-Displayed Nano Phages: Antimicrobial Function against Methicillin-Resistant Staphylococcus aureus (MRSA).Avicenna J Med Biotechnol. 2020 Apr-Jun;12(2):85-90. Avicenna J Med Biotechnol. 2020. PMID: 32431792 Free PMC article.
-
The untapped potential of phage model systems as therapeutic agents.Virus Evol. 2024 Jan 13;10(1):veae007. doi: 10.1093/ve/veae007. eCollection 2024. Virus Evol. 2024. PMID: 38361821 Free PMC article.
References
-
- Harrington J, Richard Vile G, Hardev S, Pandha K. Viral Therapy of Cancer. John Wiley & Sons, Ltd; 2008.
-
- Taira K, Kataoka K, Niidome T. Non-viral Gene Therapy Gene Design and Delivery. Springer-Verlag Tokyo; 2005.
-
- Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. European Journal of Immunology. 1999;29:29. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

