Molecular mechanism of MLL PHD3 and RNA recognition by the Cyp33 RRM domain
- PMID: 20460131
- PMCID: PMC3204800
- DOI: 10.1016/j.jmb.2010.04.067
Molecular mechanism of MLL PHD3 and RNA recognition by the Cyp33 RRM domain
Abstract
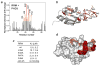
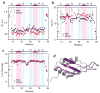
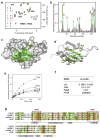
The nuclear protein cyclophilin 33 (Cyp33) is a peptidyl-prolyl cis-trans isomerase that catalyzes cis-trans isomerization of the peptide bond preceding a proline and promotes folding and conformational changes in folded and unfolded proteins. The N-terminal RNA-recognition motif (RRM) domain of Cyp33 has been found to associate with the third plant homeodomain (PHD3) finger of the mixed lineage leukemia (MLL) proto-oncoprotein and a poly(A) RNA sequence. Here, we report a 1.9 A resolution crystal structure of the RRM domain of Cyp33 and describe the molecular mechanism of PHD3 and RNA recognition. The Cyp33 RRM domain folds into a five-stranded antiparallel beta-sheet and two alpha-helices. The RRM domain, but not the catalytic module of Cyp33, binds strongly to PHD3, exhibiting a 2 muM affinity as measured by isothermal titration calorimetry. NMR chemical shift perturbation (CSP) analysis and dynamics data reveal that the beta strands and the beta2-beta3 loop of the RRM domain are involved in the interaction with PHD3. Mutations in the PHD3-binding site or deletions in the beta2-beta3 loop lead to a significantly reduced affinity or abrogation of the interaction. The RNA-binding pocket of the Cyp33 RRM domain, mapped on the basis of NMR CSP and mutagenesis, partially overlaps with the PHD3-binding site, and RNA association is abolished in the presence of MLL PHD3. Full-length Cyp33 acts as a negative regulator of MLL-induced transcription and reduces the expression levels of MLL target genes MEIS1 and HOXA9. Together, these in vitro and in vivo data provide insight into the multiple functions of Cyp33 RRM and suggest a Cyp33-dependent mechanism for regulating the transcriptional activity of MLL.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
The PHD3 domain of MLL acts as a CYP33-regulated switch between MLL-mediated activation and repression.Biochemistry. 2010 Aug 10;49(31):6576-86. doi: 10.1021/bi1009387. Biochemistry. 2010. PMID: 20677832 Free PMC article.
-
Cyp33 binds AU-rich RNA motifs via an extended interface that competitively disrupts the gene repressive Cyp33-MLL1 interaction in vitro.PLoS One. 2021 Feb 19;16(2):e0237956. doi: 10.1371/journal.pone.0237956. eCollection 2021. PLoS One. 2021. PMID: 33606679 Free PMC article.
-
Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression.Cell. 2010 Jun 25;141(7):1183-94. doi: 10.1016/j.cell.2010.05.016. Epub 2010 Jun 10. Cell. 2010. PMID: 20541251 Free PMC article.
-
The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression.FEBS J. 2005 May;272(9):2118-31. doi: 10.1111/j.1742-4658.2005.04653.x. FEBS J. 2005. PMID: 15853797 Review.
-
Integrated structural biology to unravel molecular mechanisms of protein-RNA recognition.Methods. 2017 Apr 15;118-119:119-136. doi: 10.1016/j.ymeth.2017.03.015. Epub 2017 Mar 16. Methods. 2017. PMID: 28315749 Review.
Cited by
-
Diverse functions of PHD fingers of the MLL/KMT2 subfamily.Biochim Biophys Acta. 2014 Feb;1843(2):366-71. doi: 10.1016/j.bbamcr.2013.11.016. Epub 2013 Nov 28. Biochim Biophys Acta. 2014. PMID: 24291127 Free PMC article. Review.
-
The basic tilted helix bundle domain of the prolyl isomerase FKBP25 is a novel double-stranded RNA binding module.Nucleic Acids Res. 2017 Nov 16;45(20):11989-12004. doi: 10.1093/nar/gkx852. Nucleic Acids Res. 2017. PMID: 29036638 Free PMC article.
-
Systematic Classification of Mixed-Lineage Leukemia Fusion Partners Predicts Additional Cancer Pathways.Ann Lab Med. 2016 Mar;36(2):85-100. doi: 10.3343/alm.2016.36.2.85. Ann Lab Med. 2016. PMID: 26709255 Free PMC article. Review.
-
Insights into the mechanisms driven by H3K4 KMTs in pancreatic cancer.Biochem J. 2024 Aug 7;481(15):983-997. doi: 10.1042/BCJ20230374. Biochem J. 2024. PMID: 39078225 Free PMC article. Review.
-
Inhibition of class I HDACs abrogates the dominant effect of MLL-AF4 by activation of wild-type MLL.Oncogenesis. 2014 Nov 17;3(11):e127. doi: 10.1038/oncsis.2014.39. Oncogenesis. 2014. PMID: 25402609 Free PMC article.
References
-
- Mi H, Kops O, Zimmermann E, Jaschke A, Tropschug M. A nuclear RNA-binding cyclophilin in human T cells. FEBS Lett. 1996;398:201–5. - PubMed
-
- Wang XJ, Etzkorn FA. Peptidyl-prolyl isomerase inhibitors. Biopolymers. 2006;84:125–46. - PubMed
-
- Min L, Fulton DB, Andreotti AH. A case study of proline isomerization in cell signaling. Front Biosci. 2005;10:385–97. - PubMed
-
- Wang Y, Han R, Zhang W, Yuan Y, Zhang X, Long Y, Mi H. Human CyP33 binds specifically to mRNA and binding stimulates PPIase activity of hCyP33. FEBS Lett. 2008;582:835–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

