Sustained release nitric oxide from long-lived circulating nanoparticles
- PMID: 20460149
- PMCID: PMC2903640
- DOI: 10.1016/j.freeradbiomed.2010.04.034
Sustained release nitric oxide from long-lived circulating nanoparticles
Abstract
The current limitations of nitric oxide (NO) delivery systems have stimulated an extraordinary interest in the development of compounds that generate NO in a controlled and sustained manner with a heavy emphasis on the treatment of cardiovascular disease states. This work describes the positive physiological response to the infusion of NO-releasing nanoparticles prepared using a new platform based on hydrogel/glass hybrid nanoparticles. When exposed to moisture, these nanoparticles slowly release therapeutic levels of NO, previously generated through thermal reduction of nitrite to NO trapped within the dry particles. The controlled and sustained release of NO observed from these nanoparticles (NO-np) is regulated by its hydration over extended periods of time. In a dose-dependent manner, circulating NO-np both decreased mean arterial blood pressure and increased exhaled concentrations of NO over a period of several hours. Circulating NO-np induced vasodilatation and increased microvascular perfusion during their several hour circulation lifetime. Control nanoparticles (control-np; without nitrite) did not induce changes in arterial pressure, although a decrease in the number of capillaries perfused and an increase in leukocyte rolling and immobilization in the microcirculation were observed. The NO released by the NO-np prevents the inflammatory response observed after infusion of control-np. These data suggest that NO release from NO-np is advantageous relative to other NO-releasing compounds, because it does not depend on chemical decomposition or enzymatic catalysis; it is only determined by the rate of hydration. Based on the observed physiological properties, NO-np has clear potential as a therapeutic agent and as a research tool to increase our understanding of NO signaling mechanisms within the vasculature.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
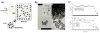
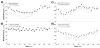
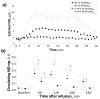
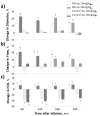

Comment in
-
NO gets a test ride on high-tech transporting nanodevices: A commentary on "Sustained-release nitric oxide from long-lived circulating nanoparticles".Free Radic Biol Med. 2010 Aug 15;49(4):528-9. doi: 10.1016/j.freeradbiomed.2010.05.022. Free Radic Biol Med. 2010. PMID: 20639121 No abstract available.
References
-
- Zacharia IG, Deen WM. Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng. 2005;33:214–222. - PubMed
-
- Moncada S, Palmer MJ, Higgs EA. Nitric Oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–134. - PubMed
-
- Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res. 1998;35:73–84. - PubMed
-
- Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. - PubMed
-
- Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

