Physiological and pharmacological characterizations of the larval Anopheles albimanus rectum support a change in protein distribution and/or function in varying salinities
- PMID: 20460167
- PMCID: PMC2904869
- DOI: 10.1016/j.cbpa.2010.05.002
Physiological and pharmacological characterizations of the larval Anopheles albimanus rectum support a change in protein distribution and/or function in varying salinities
Abstract
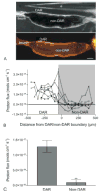
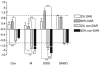
Ion regulation is a biological process crucial to the survival of mosquito larvae and a major organ responsible for this regulation is the rectum. The recta of anopheline larvae are distinct from other subfamilies of mosquitoes in several ways, yet have not yet been characterized extensively. Here we characterize the two major cell types of the anopheline rectum, DAR and non-DAR cells, using histological, physiological, and pharmacological analyses. Proton flux was measured at the basal membrane of 2%- and 50%-artificial sea water-reared An. albimanus larvae using self-referencing ion-selective microelectrodes, and the two cell types were found to differ in basal membrane proton flux. Additionally, differences in the response of that flux to pharmacological inhibitors in larvae reared in 2% versus 50% ASW indicate changes in protein function between the two rearing conditions. Finally, histological analyses suggest that the non-DAR cells are structurally suited for mediating ion transport. These data support a model of rectal ion regulation in which the non-DAR cells have a resorptive function in freshwater-reared larvae and a secretive function in saline water-reared larvae. In this way, anopheline larvae may adapt to varying salinities.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Beyenbach KW. Mechanism and regulation of electrolyte transport in Malpighian tubules. J Insect Physiol. 1995;4:197–207.
-
- Boron WF. Sodium-coupled bicarbonate transporters. JOP. 2001;2:176–181. - PubMed
-
- Bradley TJ. Evidence for hypo- and hyperosmotic regulation in the larvae of an anopheline mosquito. Am Zool. 1987a;27(4):30A.
-
- Bradley TJ. Physiology of osmoregulation in mosquitoes. Annu Rev Entomol. 1987b;32:439–462. - PubMed
-
- Bradley TJ. The role of physiological capacity, morphology, and phylogeny in determining habitat use in mosquitoes. In: Wainwright PC, Reilly SM, editors. Ecological Morphology. The University of Chicago Press; Chicago and London: 1994. pp. 303–318.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

