Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors
- PMID: 20460369
- PMCID: PMC2867323
- DOI: 10.1242/dev.040410
Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors
Abstract
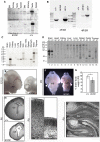
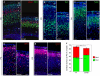
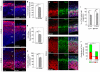
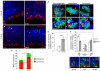
Microcephaly affects approximately 1% of the population and is associated with mental retardation, motor defects and, in some cases, seizures. We analyzed the mechanisms underlying brain size determination in a mouse model of human microcephaly. The Hertwig's anemia (an) mutant shows peripheral blood cytopenias, spontaneous aneuploidy and a predisposition to hematopoietic tumors. We found that the an mutation is a genomic inversion of exon 4 of Cdk5rap2, resulting in an in-frame deletion of exon 4 from the mRNA. The finding that CDK5RAP2 human mutations cause microcephaly prompted further analysis of Cdk5rap2(an/an) mice and we demonstrated that these mice exhibit microcephaly comparable to that of the human disease, resulting from striking neurogenic defects that include proliferative and survival defects in neuronal progenitors. Cdk5rap2(an/an) neuronal precursors exit the cell cycle prematurely and many undergo apoptosis. These defects are associated with impaired mitotic progression coupled with abnormal mitotic spindle pole number and mitotic orientation. Our findings suggest that the reduction in brain size observed in humans with mutations in CDK5RAP2 is associated with impaired centrosomal function and with changes in mitotic spindle orientation during progenitor proliferation.
Figures
References
-
- Angevine J. B., Jr, Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766-768 - PubMed
-
- Bani-Yaghoub M., Tremblay R. G., Lei J. X., Zhang D., Zurakowski B., Sandhu J. K., Smith B., Ribecco-Lutkiewicz M., Kennedy J., Walker P. R., et al. (2006). Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 295, 52-66 - PubMed
-
- Barker J. E., Bernstein S. E. (1983). Hertwig's anemia: characterization of the stem cell defect. Blood 61, 765-769 - PubMed
-
- Barker J. E., Deveau S. A., Compton S. T., Fancher K., Eppig J. T. (2005). High incidence, early onset of histiocytic sarcomas in mice with Hertwig's anemia. Exp. Hematol. 33, 1118-1129 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

