Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and ERK MAPKs, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa
- PMID: 20460375
- PMCID: PMC2903409
- DOI: 10.1074/jbc.M109.098566
Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and ERK MAPKs, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa
Abstract
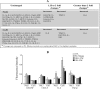
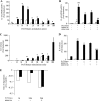
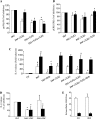
In cystic fibrosis (CF), the absence of functional cystic fibrosis transmembrane conductance regulator (CFTR) translates into chronic bacterial infection, excessive inflammation, tissue damage, impaired lung function and eventual death. Understanding the mechanisms underlying this vicious circle of inflammation is important to design better therapies for CF. We found in CF lung biopsies increased immunoreactivity for p38 MAPK activity markers. Moreover, when compared with their non-CF counterpart, airway epithelial cells expressing the most common mutation in CF (CFTRDeltaF508) were more potent at inducing neutrophil chemotaxis through increased interleukin (IL)-6 synthesis when challenged with Pseudomonas aeruginosa diffusible material. We then discovered that in CFTRDeltaF508 cells, the p38 and ERK MAPKs are hyperactivated in response to P. aeruginosa diffusible material, leading to increased IL-6 mRNA expression and stability. Moreover, although TLR5 contributes to p38 MAPK activation upon P. aeruginosa challenge, it only played a weak role in IL-6 synthesis. Instead, we found that the production of reactive oxygen species is essential for IL-6 synthesis in response to P. aeruginosa diffusible material. Finally, we uncovered that in CFTRDeltaF508 cells, the extracellular glutathione levels are decreased, leading to a greater sensitivity to reactive oxygen species, providing an explanation for the hyperactivation of the p38 and ERK MAPKs and increased IL-6 synthesis. Taken together, our study has characterized a mechanism whereby the CFTRDeltaF508 mutation in airway epithelial cells contributes to increase inflammation of the airways.
Figures



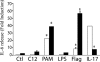
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

