Expression of LIGHT/TNFSF14 combined with vaccination against human papillomavirus Type 16 E7 induces significant tumor regression
- PMID: 20460520
- PMCID: PMC2873073
- DOI: 10.1158/0008-5472.CAN-09-3773
Expression of LIGHT/TNFSF14 combined with vaccination against human papillomavirus Type 16 E7 induces significant tumor regression
Abstract
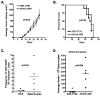
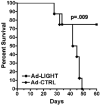
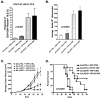
LIGHT, a ligand for the lymphotoxin-beta receptor, establishes lymphoid-like tissues inside tumor sites and recruits naïve T cells into the tumor. However, whether these infiltrating T cells are specific for tumor antigens is not known. We hypothesized that therapy with LIGHT can expand functional tumor-specific CD8(+) T cells that can be boosted using HPV16E6E7-Venezuelan equine encephalitis virus replicon particles (HPV16-VRP) and that this combined therapy can eradicate human papillomavirus 16 (HPV16)-induced tumors. Our data show that forced expression of LIGHT in tumors results in an increase in expression of IFNgamma and chemoattractant cytokines such as interleukin-1a, MIG, and macrophage inflammatory protein-2 within the tumor and that this tumor microenvironment correlates with an increase in frequency of tumor-infiltrating CD8(+) T cells. Forced expression of LIGHT also results in the expansion of functional T cells that recognize multiple tumor antigens, including HPV16 E7, and these T cells prevent the outgrowth of tumors on secondary challenge. Subsequent boosting of E7-specific T cells by vaccination with HPV16-VRP significantly increases their frequency in both the periphery and the tumor and leads to the eradication of large well-established tumors, for which either treatment alone is not successful. These data establish the safety of Ad-LIGHT as a therapeutic intervention in preclinical studies and suggest that patients with HPV16(+) tumors may benefit from combined immunotherapy with LIGHT and antigen-specific vaccination.
(c)2010 AACR.
Figures



Similar articles
-
Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA.Cancer Res. 2001 Nov 1;61(21):7861-7. Cancer Res. 2001. PMID: 11691804
-
Bivalent therapeutic vaccine against HPV16/18 genotypes consisting of a fusion protein between the extra domain A from human fibronectin and HPV16/18 E7 viral antigens.J Immunother Cancer. 2020 Jun;8(1):e000704. doi: 10.1136/jitc-2020-000704. J Immunother Cancer. 2020. PMID: 32581060 Free PMC article.
-
Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes.Vaccine. 2004 Jan 2;22(3-4):520-7. doi: 10.1016/j.vaccine.2003.07.003. Vaccine. 2004. PMID: 14670335
-
TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers.Cancer Immunol Immunother. 2012 Aug;61(8):1307-17. doi: 10.1007/s00262-012-1259-8. Epub 2012 Apr 22. Cancer Immunol Immunother. 2012. PMID: 22527249 Free PMC article. Review.
-
Inducing Immunity Where It Matters: Orthotopic HPV Tumor Models and Therapeutic Vaccinations.Front Immunol. 2020 Aug 14;11:1750. doi: 10.3389/fimmu.2020.01750. eCollection 2020. Front Immunol. 2020. PMID: 32922389 Free PMC article. Review.
Cited by
-
The role of costimulatory molecules in glioma biology and immune microenvironment.Front Genet. 2022 Nov 9;13:1024922. doi: 10.3389/fgene.2022.1024922. eCollection 2022. Front Genet. 2022. PMID: 36437961 Free PMC article.
-
In vivo detection of antigen-specific CD8+ T cells by immuno-positron emission tomography.Nat Methods. 2020 Oct;17(10):1025-1032. doi: 10.1038/s41592-020-0934-5. Epub 2020 Sep 14. Nat Methods. 2020. PMID: 32929269 Free PMC article.
-
The 12-CK Score: Global Measurement of Tertiary Lymphoid Structures.Front Immunol. 2021 Jun 29;12:694079. doi: 10.3389/fimmu.2021.694079. eCollection 2021. Front Immunol. 2021. PMID: 34267760 Free PMC article. Review.
-
Identification of a Costimulatory Molecule Gene Signature to Predict Survival and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma.Front Cell Dev Biol. 2021 Aug 9;9:695533. doi: 10.3389/fcell.2021.695533. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34434928 Free PMC article.
-
Replicon RNA vaccines: design, delivery, and immunogenicity in infectious diseases and cancer.J Hematol Oncol. 2025 Apr 17;18(1):43. doi: 10.1186/s13045-025-01694-2. J Hematol Oncol. 2025. PMID: 40247301 Free PMC article. Review.
References
-
- Nasiell K, Roger V, Nasiell M. Behavior of mild cervical dysplasia during long-term follow-up. Obstet Gynecol. 1986;67:665–9. - PubMed
-
- Benton C, Shyahidullah H, Hunter JAM. Human papillomavirus in the immunocompromised. Papillomavirus Rep. 1999;3:23–6.
-
- Laga M, Icenogle JP, Marsella R, Manoka AT, Nzila N, Ryder RW, et al. Genital papillomavirus infection and cervical dysplasia--opportunistic complications of HIV infection. IntJCancer. 1992;50:45–8. - PubMed
-
- Maiman M, Fruchter RG, Serur E, Remy JC, Feuer G, Boyce J. Human immunodeficiency virus infection and cervical neoplasia. GynecolOncol. 1990;38:377–82. - PubMed
-
- Rudlinger R, Smith IW, Bunney MH, Hunter JA. Human papillomavirus infections in a group of renal transplant recipients. BrJDermatol. 1986;115:681–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

