Structural basis for the assembly and gate closure mechanisms of the Mycobacterium tuberculosis 20S proteasome
- PMID: 20461058
- PMCID: PMC2892373
- DOI: 10.1038/emboj.2010.95
Structural basis for the assembly and gate closure mechanisms of the Mycobacterium tuberculosis 20S proteasome
Abstract
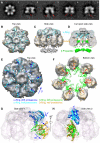
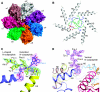
Mycobacterium tuberculosis (Mtb) possesses a proteasome system analogous to the eukaryotic ubiquitin-proteasome pathway. Mtb requires the proteasome to resist killing by the host immune system. The detailed assembly process and the gating mechanism of Mtb proteasome have remained unknown. Using cryo-electron microscopy and X-ray crystallography, we have obtained structures of three Mtb proteasome assembly intermediates, showing conformational changes during assembly, and explaining why the beta-subunit propeptide inhibits rather than promotes assembly. Although the eukaryotic proteasome core particles close their protein substrate entrance gates with different amino terminal peptides of the seven alpha-subunits, it has been unknown how a prokaryotic proteasome might close the gate at the symmetry axis with seven identical peptides. We found in the new Mtb proteasome crystal structure that the gate is tightly sealed by the seven identical peptides taking on three distinct conformations. Our work provides the structural bases for assembly and gating mechanisms of the Mtb proteasome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Adams J (2003) The proteasome: structure, function, and role in the cell. Cancer Treat Rev 29: 3–9 - PubMed
-
- Borissenko L, Groll M (2007) 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem Rev 107: 687–717 - PubMed
-
- Brunger AT (2007) Version 1.2 of the Crystallography and NMR system. Nat Protoc 2: 2728–2733 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

