Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms
- PMID: 20461061
- PMCID: PMC2911264
- DOI: 10.1038/mt.2010.85
Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms
Abstract
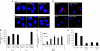
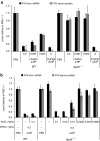
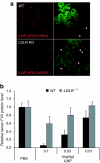
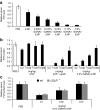
Lipid nanoparticles (LNPs) have proven to be highly efficient carriers of short-interfering RNAs (siRNAs) to hepatocytes in vivo; however, the precise mechanism by which this efficient delivery occurs has yet to be elucidated. We found that apolipoprotein E (apoE), which plays a major role in the clearance and hepatocellular uptake of physiological lipoproteins, also acts as an endogenous targeting ligand for ionizable LNPs (iLNPs), but not cationic LNPs (cLNPs). The role of apoE was investigated using both in vitro studies employing recombinant apoE and in vivo studies in wild-type and apoE(-/-) mice. Receptor dependence was explored in vitro and in vivo using low-density lipoprotein receptor (LDLR(-/-))-deficient mice. As an alternative to endogenous apoE-based targeting, we developed a targeting approach using an exogenous ligand containing a multivalent N-acetylgalactosamine (GalNAc)-cluster, which binds with high affinity to the asialoglycoprotein receptor (ASGPR) expressed on hepatocytes. Both apoE-based endogenous and GalNAc-based exogenous targeting appear to be highly effective strategies for the delivery of iLNPs to liver.
Figures

References
-
- de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. - PubMed
-
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. - PubMed
-
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

