SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells
- PMID: 20461063
- PMCID: PMC2911258
- DOI: 10.1038/mt.2010.77
SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells
Abstract
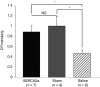
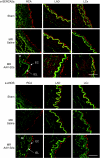
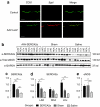
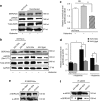
Congestive heart failure (HF) is associated with impaired endothelium-dependent nitric oxide-mediated vasodilatation. The aim of this study was to examine the effects of sarco/endoplasmic reticulum (ER) Ca(2+)-ATPase 2a (SERCA2a) gene transfer on endothelial function in a swine HF model. Two months after the creation of mitral regurgitation to induce HF, the animals underwent intracoronary injection of adeno-associated virus (AAV) carrying SERCA2a (n = 7) or saline (n = 6). At 4 months, coronary flow (CF) was measured in the mid-portion of the left anterior descending (LAD) artery. In the failing animals, CF was decreased significantly; SERCA2a gene transfer rescued CF to levels observed in sham-group [ml/min/g, 0.47 +/- 0.064 saline versus 0.89 +/- 0.116, SERCA2a; P < 0.05; 1.00 +/- 0. 185 sham P = NS (nonsignificant)]. In coronary arteries from HF animals, SERCA2a and endothelial isoform of nitric oxide synthase (eNOS) protein expression were decreased, but restored to normal levels by SERCA2a gene transfer. In human coronary artery endothelial cells (HCAECs), SERCA2a overexpression increased eNOS expression, phosphorylation, eNOS promoter activity, Ca(2+) storage capacity, and enhanced histamine-induced calcium oscillations, eNOS activity, and cyclic guanosine monophosphate (cGMP) production. Thus, SERCA2a gene transfer increases eNOS expression and activity by modulating calcium homeostasis to improve CF. These findings suggest that SERCA2a gene transfer improves vascular reactivity in the setting of HF.
Figures




References
-
- Drexler H, Hayoz D, Münzel T, Just H, Zelis R., and, Brunner HR. Endothelial function in congestive heart failure. Am Heart J. 1993;126:761–764. - PubMed
-
- Kubo SH, Rector TS, Bank AJ, Williams RE., and, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. - PubMed
-
- Mohri M, Egashira K, Tagawa T, Kuga T, Tagawa H, Harasawa Y, et al. Basal release of nitric oxide is decreased in the coronary circulation in patients with heart failure. Hypertension. 1997;30:50–56. - PubMed
-
- Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–779. - PubMed
-
- Zhao G, Shen W, Xu X, Ochoa M, Bernstein R., and, Hintze TH. Selective impairment of vagally mediated, nitric oxide-dependent coronary vasodilation in conscious dogs after pacing-induced heart failure. Circulation. 1995;91:2655–2663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL071763/HL/NHLBI NIH HHS/United States
- K01 HL103176/HL/NHLBI NIH HHS/United States
- HL77101/HL/NHLBI NIH HHS/United States
- HL071763/HL/NHLBI NIH HHS/United States
- R01 HL026057/HL/NHLBI NIH HHS/United States
- HL64018/HL/NHLBI NIH HHS/United States
- HL083156/HL/NHLBI NIH HHS/United States
- R01 HL083156/HL/NHLBI NIH HHS/United States
- R01 HL078691/HL/NHLBI NIH HHS/United States
- R01 HL064018/HL/NHLBI NIH HHS/United States
- HL057263/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
- HL26057/HL/NHLBI NIH HHS/United States
- R37 HL026057/HL/NHLBI NIH HHS/United States
- K02 HL081110/HL/NHLBI NIH HHS/United States
- R01 HL080498/HL/NHLBI NIH HHS/United States
- HL700819/HL/NHLBI NIH HHS/United States
- HL080498/HL/NHLBI NIH HHS/United States
- R01 HL057263/HL/NHLBI NIH HHS/United States
- HL81110/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

