The differential imaging features of fat-containing tumors in the peritoneal cavity and retroperitoneum: the radiologic-pathologic correlation
- PMID: 20461188
- PMCID: PMC2864861
- DOI: 10.3348/kjr.2010.11.3.333
The differential imaging features of fat-containing tumors in the peritoneal cavity and retroperitoneum: the radiologic-pathologic correlation
Abstract
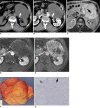
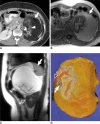
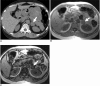
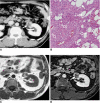
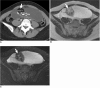
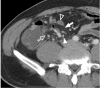
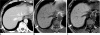
There are a variety of fat-containing lesions that can arise in the intraperitoneal cavity and retroperitoneal space. Some of these fat-containing lesions, such as liposarcoma and retroperitoneal teratoma, have to be resected, although resection can be deferred for others, such as adrenal adenoma, myelolipoma, angiomyolipoma, ovarian teratoma, and lipoma, until the lesions become large or symptomatic. The third group tumors (i.e., mesenteric panniculitis and pseudolipoma of Glisson's capsule) require medical treatment or no treatment at all. Identifying factors such as whether the fat is macroscopic or microscopic within the lesion, the origin of the lesions, and the presence of combined calcification is important for narrowing the differential diagnosis. The development and wide-spread use of modern imaging modalities make identification of these factors easier so narrowing the differential diagnosis is possible. At the same time, lesions that do not require immediate treatment are being incidentally found at an increasing rate with these same imaging techniques. Thus, the questions about the treatment methods have become increasingly important. Classifying lesions in terms of the necessity of performing surgical treatment can provide important information to clinicians, and this is the one of a radiologist's key responsibilities.
Keywords: Abdominal cavity; Magnetic resonance (MR); Neoplasm, adipose tissue; Retroperitoneal space; Tomography, spiral computed.
Figures





References
-
- Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics. 2005;25:321–331. - PubMed
-
- Pereira JM, Sirlin CB, Pinto PS, Casola G. CT and MR imaging of extrahepatic fatty masses of the abdomen and pelvis: techniques, diagnosis, differential diagnosis, and pitfalls. Radiographics. 2005;25:69–85. - PubMed
-
- Israel GM, Bosniak MA, Slywotzky CM, Rosen RJ. CT differentiation of large exophytic renal angiomyolipomas and perirenal liposarcomas. AJR Am J Roentgenol. 2002;179:769–773. - PubMed
-
- Weiss SW. Lipomatous tumors. In: Weiss SW, Brooks JSJ, editors. Soft tissue tumors. Baltimore: Williams & Wilkins; 1996. pp. 207–251.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

