Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice
- PMID: 20461230
- PMCID: PMC2866455
- DOI: 10.3389/fncir.2010.00009
Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice
Abstract
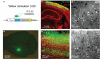
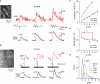
Fluorescent calcium (Ca(2+)) indicator proteins (FCIPs) are promising tools for functional imaging of cellular activity in living animals. However, they have still not reached their full potential for in vivo imaging of neuronal activity due to limitations in expression levels, dynamic range, and sensitivity for reporting action potentials. Here, we report that viral expression of the ratiometric Ca(2+) sensor yellow cameleon 3.60 (YC3.60) in pyramidal neurons of mouse barrel cortex enables in vivo measurement of neuronal activity with high dynamic range and sensitivity across multiple spatial scales. By combining juxtacellular recordings and two-photon imaging in vitro and in vivo, we demonstrate that YC3.60 can resolve single action potential (AP)-evoked Ca(2+) transients and reliably reports bursts of APs with negligible saturation. Spontaneous and whisker-evoked Ca(2+) transients were detected in individual apical dendrites and somata as well as in local neuronal populations. Moreover, bulk measurements using wide-field imaging or fiber-optics revealed sensory-evoked YC3.60 signals in large areas of the barrel field. Fiber-optic recordings in particular enabled measurements in awake, freely moving mice and revealed complex Ca(2+) dynamics, possibly reflecting different behavior-related brain states. Viral expression of YC3.60 - in combination with various optical techniques - thus opens a multitude of opportunities for functional studies of the neural basis of animal behavior, from dendrites to the levels of local and large-scale neuronal populations.
Keywords: adeno-associated virus; barrel cortex; calcium; neocortex; two-photon microscopy; yellow cameleon.
Figures

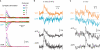



Similar articles
-
Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex.J Physiol. 2000 Dec 15;529 Pt 3(Pt 3):625-46. doi: 10.1111/j.1469-7793.2000.00625.x. J Physiol. 2000. PMID: 11118494 Free PMC article.
-
Simultaneous Two-Photon Voltage or Calcium Imaging and Multi-Channel Local Field Potential Recordings in Barrel Cortex of Awake and Anesthetized Mice.Front Neurosci. 2021 Nov 11;15:741279. doi: 10.3389/fnins.2021.741279. eCollection 2021. Front Neurosci. 2021. PMID: 34867155 Free PMC article.
-
Back-propagating action potentials mediate calcium signalling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex.J Physiol. 2001 Aug 15;535(Pt 1):17-31. doi: 10.1111/j.1469-7793.2001.t01-1-00017.x. J Physiol. 2001. PMID: 11507155 Free PMC article.
-
In vivo deep two-photon imaging of neural circuits with the fluorescent Ca2+ indicator Cal-590.J Physiol. 2017 May 15;595(10):3097-3105. doi: 10.1113/JP272790. Epub 2017 Feb 5. J Physiol. 2017. PMID: 27995645 Free PMC article. Review.
-
Wide-Field Calcium Imaging of Neuronal Network Dynamics In Vivo.Biology (Basel). 2022 Nov 1;11(11):1601. doi: 10.3390/biology11111601. Biology (Basel). 2022. PMID: 36358302 Free PMC article. Review.
Cited by
-
Putting a finishing touch on GECIs.Front Mol Neurosci. 2014 Nov 18;7:88. doi: 10.3389/fnmol.2014.00088. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25477779 Free PMC article. Review.
-
Gradient Index Microlens Implanted in Prefrontal Cortex of Mouse Does Not Affect Behavioral Test Performance over Time.PLoS One. 2016 Jan 22;11(1):e0146533. doi: 10.1371/journal.pone.0146533. eCollection 2016. PLoS One. 2016. PMID: 26799938 Free PMC article.
-
Spike rate inference from mouse spinal cord calcium imaging data.J Neurosci. 2025 Mar 24;45(18):e1187242025. doi: 10.1523/JNEUROSCI.1187-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40127941 Free PMC article.
-
Quantitative comparison of genetically encoded Ca indicators in cortical pyramidal cells and cerebellar Purkinje cells.Front Cell Neurosci. 2011 Sep 29;5:18. doi: 10.3389/fncel.2011.00018. eCollection 2011. Front Cell Neurosci. 2011. PMID: 21994490 Free PMC article.
-
Imaging neural activity using Thy1-GCaMP transgenic mice.Neuron. 2012 Oct 18;76(2):297-308. doi: 10.1016/j.neuron.2012.07.011. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083733 Free PMC article.
References
-
- Berger T., Borgdorff A., Crochet S., Neubauer F. B., Lefort S., Fauvet B., Ferezou I., Carleton A., Lüscher H. R., Petersen C. C. (2007). Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J. Neurophysiol. 97, 3751–376210.1152/jn.01178.2006 - DOI - PubMed
-
- Chattopadhyaya B., Di Cristo G., Higashiyama H., Knott G. W., Kuhlman S. J., Welker E., Huang Z. J. (2004). Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–961110.1523/JNEUROSCI.1851-04.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

