Hyperquenching for protein cryocrystallography
- PMID: 20461232
- PMCID: PMC2866519
- DOI: 10.1107/S0021889806037484
Hyperquenching for protein cryocrystallography
Abstract
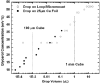
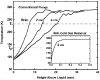
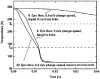
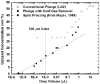
When samples having volumes characteristic of protein crystals are plunge cooled in liquid nitrogen or propane, most cooling occurs in the cold gas layer above the liquid. By removing this cold gas layer, cooling rates for small samples and modest plunge velocities are increased to 1.5 × 10(4) K s(-1), with increases of a factor of 100 over current best practice possible with 10 μm samples. Glycerol concentrations required to eliminate water crystallization in protein-free aqueous mixtures drop from ∼28% w/v to as low as 6% w/v. These results will allow many crystals to go from crystallization tray to liquid cryogen to X-ray beam without cryoprotectants. By reducing or eliminating the need for cryoprotectants in growth solutions, they may also simplify the search for crystallization conditions and for optimal screens. The results presented here resolve many puzzles, such as why plunge cooling in liquid nitrogen or propane has, until now, not yielded significantly better diffraction quality than gas-stream cooling.
Figures

References
-
- Bruggeller P, Mayer E. Nature (London) 1980;288:569–571.
-
- Chang ZH, Baust JG. J Microsc. 1991;161:435–444. - PubMed
-
- Chayen NE, Boggon TJ, Cassetta A, Deacon A, Gleichmann T, Habash J, Harrop SJ, Helliwell JR, Nieh YP, Peterson MR, Raftery J, Snell EH, Hadener A, Niemann AC, Siddons DP, Stojanoff V, Thompson AW, Ursby T, Wulff M. Q Rev Biophys. 1996;29:227–278. - PubMed
-
- Chinte U, Shah B, DeWitt K, Kirschbaum K, Pinkerton AA, Schall C. J Appl Cryst. 2005;38:412–419.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
