Orchestration of "presto" and "largo" synchrony in up-down activity of cortical networks
- PMID: 20461235
- PMCID: PMC2866559
- DOI: 10.3389/fncir.2010.00011
Orchestration of "presto" and "largo" synchrony in up-down activity of cortical networks
Abstract
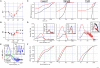
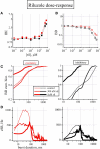
It has been demonstrated using single-cell and multiunit electrophysiology in layer III entorhinal cortex and disinhibited hippocampal CA3 slices that the balancing of the up-down activity is characterized by both GABA(A) and GABA(B) mechanisms. Here we report novel results obtained using multi-electrode array (60 electrodes) simultaneous recordings from reverberating postnatal neocortical networks containing 19.2 +/- 1.4% GABAergic neurons, typical of intact tissue. We observed that in each spontaneous active-state the total number of spikes in identified clusters of excitatory and inhibitory neurons is almost equal, thus suggesting a balanced average activity. Interestingly, in the active-state, the early phase is sustained by only 10% of the total spikes and the firing rate follows a sigmoidal regenerative mode up to peak at 35 ms with the number of excitatory spikes greater than inhibitory, therefore indicating an early unbalance. Concentration-response pharmacology of up- and down-state lifetimes in clusters of excitatory (n = 1067) and inhibitory (n = 305) cells suggests that, besides the GABA(A) and GABA(B) mechanisms, others such as GAT-1-mediated uptake, I(h), I(NaP) and I(M) ion channel activity, robustly govern both up- and down-activity. Some drugs resulted to affect up- and/or down-states with different IC(50)s, providing evidence that various mechanisms are involved. These results should reinforce not only the role of synchrony in CNS networks, but also the recognized analogies between the Hodgkin-Huxley action potential and the population bursts as basic mechanisms for originating membrane excitability and CNS network synchronization, respectively.
Keywords: GABA; IM channels; INaP channels; Ih channels; MEA recording; bursts; synchrony.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

