Immunohistochemical localization and mRNA expression of aquaporins in the macula utriculi of patients with Meniere's disease and acoustic neuroma
- PMID: 20461409
- PMCID: PMC2882038
- DOI: 10.1007/s00441-010-0975-7
Immunohistochemical localization and mRNA expression of aquaporins in the macula utriculi of patients with Meniere's disease and acoustic neuroma
Abstract
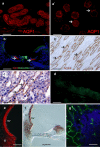
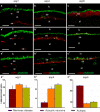
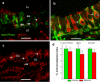
Meniere's disease is nearly invariably associated with endolymphatic hydrops (the net accumulation of water in the inner ear endolymphatic space). Vestibular maculae utriculi were acquired from patients undergoing surgery for Meniere's disease and acoustic neuroma and from autopsy (subjects with normal hearing and balance). Quantitative immunostaining was conducted with antibodies against aquaporins (AQPs) 1, 4, and 6, Na(+)K(+)ATPase, Na(+)K(+)2Cl co-transporter (NKCC1), and alpha-syntrophin. mRNA was extracted from the surgically acquired utricles from subjects with Meniere's disease and acoustic neuroma to conduct quantitative real-time reverse transcription with polymerase chain reaction for AQP1, AQP4, and AQP6. AQP1 immunoreactivity (-IR) was located in blood vessels and fibrocytes in the underlying stroma, without any apparent alteration in Meniere's specimens when compared with acoustic neuroma and autopsy specimens. AQP4-IR localized to the epithelial basolateral supporting cells in Meniere's disease, acoustic neuroma, and autopsy. In specimens from subjects with Meniere's disease, AQP4-IR was significantly decreased compared with autopsy and acoustic neuroma specimens. AQP6-IR occurred in the sub-apical vestibular supporting cells in acoustic neuroma and autopsy samples. However, in Meniere's disease specimens, AQP6-IR was significantly increased and diffusely redistributed throughout the supporting cell cytoplasm. Na(+)K(+)ATPase, NKCC1, and alpha-syntrophin were expressed within sensory epithelia and were unaltered in Meniere's disease specimens. Expression of AQP1, AQP4, or AQP6 mRNA did not differ in vestibular endorgans from patients with Meniere's disease. Changes in AQP4 (decreased) and AQP6 (increased) expression in Meniere's disease specimens suggest that the supporting cell might be a cellular target.
Figures
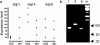
Similar articles
-
Cochlin expression in vestibular endorgans obtained from patients with Meniere's disease.Cell Tissue Res. 2012 Nov;350(2):373-84. doi: 10.1007/s00441-012-1481-x. Epub 2012 Sep 20. Cell Tissue Res. 2012. PMID: 22992960 Free PMC article.
-
Immunohistochemical localization of aquaporins in the human inner ear.Cell Tissue Res. 2007 Jun;328(3):453-60. doi: 10.1007/s00441-007-0380-z. Epub 2007 Feb 21. Cell Tissue Res. 2007. PMID: 17318586
-
Meniere's disease: histopathology, cytochemistry, and imaging.Ann N Y Acad Sci. 2015 Apr;1343:49-57. doi: 10.1111/nyas.12699. Epub 2015 Mar 12. Ann N Y Acad Sci. 2015. PMID: 25766597 Review.
-
Aquaporins as potential drug targets for Meniere's disease and its related diseases.Handb Exp Pharmacol. 2009;(190):171-84. doi: 10.1007/978-3-540-79885-9_8. Handb Exp Pharmacol. 2009. PMID: 19096777 Review.
-
Expression and translocation of aquaporin-2 in the endolymphatic sac in patients with Meniere's disease.J Neuroendocrinol. 2010 Nov;22(11):1157-64. doi: 10.1111/j.1365-2826.2010.02060.x. J Neuroendocrinol. 2010. PMID: 20722976
Cited by
-
The Price of Immune Responses and the Role of Vitamin D in the Inner Ear.Otol Neurotol. 2019 Jul;40(6):701-709. doi: 10.1097/MAO.0000000000002258. Otol Neurotol. 2019. PMID: 31194714 Free PMC article. Review.
-
Identification of new signaling components in the sensory epithelium of human saccule.Front Neurol. 2011 Aug 5;2:48. doi: 10.3389/fneur.2011.00048. eCollection 2011. Front Neurol. 2011. PMID: 21886636 Free PMC article.
-
Oxidative Stress in the Blood Labyrinthine Barrier in the Macula Utricle of Meniere's Disease Patients.Front Physiol. 2018 Sep 3;9:1068. doi: 10.3389/fphys.2018.01068. eCollection 2018. Front Physiol. 2018. PMID: 30233382 Free PMC article.
-
Immunocytochemical distribution of WARP (von Willebrand A domain-related protein) in the inner ear.Brain Res. 2011 Jan 7;1367:50-61. doi: 10.1016/j.brainres.2010.10.056. Epub 2010 Nov 18. Brain Res. 2011. PMID: 20971096 Free PMC article.
-
Corticosteroid therapy for hearing and balance disorders.Anat Rec (Hoboken). 2012 Nov;295(11):1928-43. doi: 10.1002/ar.22576. Epub 2012 Oct 8. Anat Rec (Hoboken). 2012. PMID: 23044978 Free PMC article. Review.
References
-
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. - DOI - PMC - PubMed
-
- Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129:999–1010. - PubMed
-
- Andrews JC. Intralabyrinthine fluid dynamics: Meniere’s disease. Curr Opin Otolaryngol Head Neck Surg. 2004;12:408–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

