Stereospecific synthesis of alkylidenecyclopropanes via sequential cyclopropene carbomagnesation/1,3-carbon shift
- PMID: 20462206
- PMCID: PMC2882174
- DOI: 10.1021/jo1002938
Stereospecific synthesis of alkylidenecyclopropanes via sequential cyclopropene carbomagnesation/1,3-carbon shift
Abstract
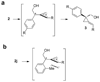
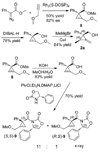
Alkylidenecyclopropanes can be synthesized from enantiomerically enriched cyclopropene derivatives with >99% stereotransfer and good to excellent yield. The protocol comprises the stereoselective reaction of Grignard reagents with 1-alkoxymethyl-3-hydroxymethylcyclopropenes and a stereospecific [1,3] carbon shift reaction.
Figures






Similar articles
-
Regio- and Diastereoselective Carbometalation Reaction of Cyclopropenes.Acc Chem Res. 2022 Oct 4;55(19):2848-2868. doi: 10.1021/acs.accounts.2c00424. Epub 2022 Sep 14. Acc Chem Res. 2022. PMID: 36102664 Free PMC article.
-
Efficient synthesis of enantiomerically pure alpha-alkyl cyclopropanecarbonitrile derivatives from pyrazolines.Org Lett. 2004 Dec 23;6(26):4945-8. doi: 10.1021/ol047937f. Org Lett. 2004. PMID: 15606106
-
An efficient, facile, and general stereoselective synthesis of heterosubstituted alkylidenecyclopropanes.Angew Chem Int Ed Engl. 2007;46(42):8039-42. doi: 10.1002/anie.200702713. Angew Chem Int Ed Engl. 2007. PMID: 17854109 No abstract available.
-
Synthesis of cyclopropene alpha-amino acids via enantioselective desymmetrization.Org Lett. 2006 Jul 6;8(14):2965-8. doi: 10.1021/ol060847l. Org Lett. 2006. PMID: 16805528
-
Stereospecific and highly stereoselective cyclopropanation reactions promoted by samarium.Chem Soc Rev. 2010 Nov;39(11):4103-13. doi: 10.1039/b915662c. Epub 2010 Aug 4. Chem Soc Rev. 2010. PMID: 20683534 Review.
Cited by
-
Regio- and Diastereoselective Carbometalation Reaction of Cyclopropenes.Acc Chem Res. 2022 Oct 4;55(19):2848-2868. doi: 10.1021/acs.accounts.2c00424. Epub 2022 Sep 14. Acc Chem Res. 2022. PMID: 36102664 Free PMC article.
-
Facially selective Cu-catalyzed carbozincation of cyclopropenes using arylzinc reagents formed by sequential I/Mg/Zn exchange.J Org Chem. 2012 Nov 2;77(21):9900-4. doi: 10.1021/jo3019076. Epub 2012 Oct 19. J Org Chem. 2012. PMID: 23035947 Free PMC article.
-
Sigmatropic rearrangements of cyclopropenylcarbinol derivatives. Access to diversely substituted alkylidenecyclopropanes.Beilstein J Org Chem. 2019 Feb 5;15:333-350. doi: 10.3762/bjoc.15.29. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30800182 Free PMC article. Review.
-
Functionalized Cyclopropenes and Methylenecyclopropenes from Dianions of 3-Hydroxymethylcyclopropenes.Synthesis (Stuttg). 2012;44(18):2843-2850. doi: 10.1055/s-0031-1290822. Synthesis (Stuttg). 2012. PMID: 23125466 Free PMC article.
References
-
- Fürstner A, Aïssa C. J. Am. Chem. Soc. 2006;128:6306. - PubMed
- Chowdhury MA, Senboku H, Tokuda M. Tetrahedron Lett. 2003;44:3329.
- Liang Y, Jiao L, Wang Y, Chen Y, Ma L, Xu J, Zhang S, Yu Z-X. Org. Lett. 2006;8:5877. - PubMed
- Taillier C, Lautens M. Org. Lett. 2007;9:591. - PubMed
- Scott ME, Lautens M. Org. Lett. 2005;7:3045. - PubMed
- Nair V, Suja TD, Mohanan K. Synthesis. 2006:2531.
-
- Siriwardana AI, Nakamura I, Yamamoto Y. J. Org. Chem. 2004;69:3202. - PubMed
- Durán J, Gulías M, Castedo L, Mascareñas JL. Org. Lett. 2005;7:5693. - PubMed
- Lautens M, Ren Y, Delanghe P. J. Am. Chem. Soc. 1994;116:8821.
- Lautens M, Ren Y. J. Am. Chem. Soc. 1996;118:9597.
- Kamikawa K, Shimizu Y, Takemoto S, Matsuzaka H. Org. Lett. 2006;8:4011. - PubMed
- de Meijere A, Kurahashi T. Angew. Chem. Int. Ed. 2005;44:7881. - PubMed
- de Meijere A, Becker H, Stolle A, Kozhushkov SI, Bes MT, Salaün J, Noltemeyer M. Chem. Eur. J. 2005;11:2471. - PubMed
- Molchanov AP, Diev VV, Magull J, Vidović D, Kozhushkov SI, de Meijere A, Kostikov RR. Eur. J. Org. Chem. 2005:593.
- Hu B, Zhu J, Xing S, Fang J, Du D, Wang Z. Chem. Eur. J. 2009;15:324. - PubMed
-
- Zhou H, Huang X, Chen W. Synlett. 2003;13:2080.
- Shi M, Shao L-X. Synlett. 2004;5:807.
- Zhu Z-b, Liu L-p, Shao L-x, Shi M. Synlett. 2007:115.
- Shi M, Jiang M, Liu L-p. Org. Biomol. Chem. 2007;5:438. - PubMed
- Shi M, Liu L-p, Tang J. Org. Lett. 2006;8:4043. - PubMed
- Chen W-l, Su C-l, Huang X. Synlett. 2006;9:1446.
- Huang X, Fu W-J. Synthesis. 2006;6:1016.
- Huang X, Yang Y. Org. Lett. 2007;9:1667. - PubMed
- Yang Y, Huang X. J. Org. Chem. 2008;73:4702. - PubMed
- Taniguchi H, Ohmura T, Suginome M. J. Am. Chem. Soc. 2009;131:11298. - PubMed
- Ohmura T, Taniguchi H, Kondo Y, Suginome M. J. Am. Chem. Soc. 2007;129:3518. - PubMed
- Hayashi N, Hirokawa Y, Shibata I, Yasuda M, Baba A. J. Am. Chem. Soc. 2008;130:2912. - PubMed
-
- Rahman W, Kuivila HG. J. Org. Chem. 1966;31:772.
- Satoh T, Saito S. Tetrahedron Lett. 2004;45:347.
- Ma S, Lu L, Zhang J. J. Am. Chem. Soc. 2004;126:9645. - PubMed
- Meijs GF, Eichinger PCH. Tetrahedron Lett. 1987;28:5559.
- Stafford JA, McMurry JE. Tetrahedron Lett. 1988;29:2531.
- Danheiser RL, Lee TW, Menichincheri M, Brunelli S, Nishiuchi M. Synlett. 1997:469.
- Bernard AM, Frongia A, Piras PP, Secci F. Synlett. 2004:1064.
- Bigeault J, Giordano L, de Riggi I, Gimbert Y, Buono G. Org. Lett. 2007;9:3567. - PubMed
- Shono T, Nagasawa T, Tsubouchi A, Takeda T. Tetrahedron Lett. 2007;48:3521.
- Thomas E, Kasatkin AN, Whitby RJ. Tetrahedron Lett. 2006;47:9181.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

