Poly(amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques
- PMID: 20462240
- PMCID: PMC2889128
- DOI: 10.1021/bc1000802
Poly(amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques
Abstract
This report presents the preparation and characterization of three [Gd-C-DOTA](-1)-dendrimer assemblies by way of analysis, NMRD spectroscopy, and photon correlation spectroscopy (PCS). The metal-ligand chelates were preformed in alcohol media prior to conjugation to generation 4, 5, and 6 PAMAM dendrimers. The dendrimer-based agents were purified by Sephadex G-25 column chromatography. The combustion analysis, SE-HPLC, and UV-vis data indicated chelate to dendrimer ratios of 28:1, 61:1 and 115:1, respectively. Molar relaxivity measured at pH 7.4, 22 degrees C, and 3 T (29.6, 49.8, and 89.1 mM(-1) s(-1)) indicated the viability of conjugates as MRI contrast agents. 1/T(1) NMRD profiles were measured at 23 degrees C and indicated that at 22 MHz the 1/T(1) reached a plateau at 60, 85, and 140 mM(-1) s(-1) for the generation 4, 5, and 6 dendrimer conjugates, respectively. The PCS data showed the respective sizes of 5.2, 6.5, and 7.8 nm for G-4, 5, and 6 conjugates.
Figures


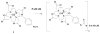
Similar articles
-
Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates.Bioorg Med Chem. 2010 Aug 15;18(16):5925-31. doi: 10.1016/j.bmc.2010.06.086. Epub 2010 Jul 8. Bioorg Med Chem. 2010. PMID: 20663676 Free PMC article.
-
Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates.J Magn Reson Imaging. 1999 Feb;9(2):348-52. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. J Magn Reson Imaging. 1999. PMID: 10077036
-
Rotational dynamics account for pH-dependent relaxivities of PAMAM dendrimeric, Gd-based potential MRI contrast agents.Chemistry. 2005 May 6;11(10):3064-76. doi: 10.1002/chem.200401326. Chemistry. 2005. PMID: 15776490
-
Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design.Bioorg Med Chem. 2016 Nov 15;24(22):5663-5684. doi: 10.1016/j.bmc.2016.09.069. Epub 2016 Oct 1. Bioorg Med Chem. 2016. PMID: 27729196 Review.
-
Synthetic approaches to heterocyclic ligands for Gd-based MRI contrast agents.Molecules. 2007 Aug 9;12(8):1771-95. doi: 10.3390/12081771. Molecules. 2007. PMID: 17960087 Free PMC article. Review.
Cited by
-
Application of Dendrimers in Anticancer Diagnostics and Therapy.Molecules. 2022 May 18;27(10):3237. doi: 10.3390/molecules27103237. Molecules. 2022. PMID: 35630713 Free PMC article. Review.
-
Preparation and In Vitro Characterization of Dendrimer-based Contrast Agents for Magnetic Resonance Imaging.J Vis Exp. 2016 Dec 4;(118):54776. doi: 10.3791/54776. J Vis Exp. 2016. PMID: 28060285 Free PMC article.
-
Preparation of cystamine core dendrimer and antibody-dendrimer conjugates for MRI angiography.Mol Pharm. 2012 Mar 5;9(3):374-81. doi: 10.1021/mp2003219. Epub 2011 Sep 21. Mol Pharm. 2012. PMID: 21882823 Free PMC article.
-
Gd3+:DOTA-Modified 2-Hydroxypropyl-β-Cyclodextrin/4-Sulfobutyl Ether-β-Cyclodextrin-Based Polyrotaxanes as Long Circulating High Relaxivity MRI Contrast Agents.Bioconjug Chem. 2018 Nov 21;29(11):3550-3560. doi: 10.1021/acs.bioconjchem.8b00525. Epub 2018 Nov 7. Bioconjug Chem. 2018. PMID: 30403467 Free PMC article.
-
Silica microparticles as a solid support for gadolinium phosphonate magnetic resonance imaging contrast agents.J Am Chem Soc. 2012 May 16;134(19):8046-9. doi: 10.1021/ja302183w. Epub 2012 May 3. J Am Chem Soc. 2012. PMID: 22545921 Free PMC article.
References
-
- Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. - PubMed
-
- Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CAS, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–871. - PubMed
-
- Gillies ER, Fréchet JMJ. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

