Pharmacological characterization of 30 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists, synthetic agonists, and the endogenous agouti-related protein antagonist
- PMID: 20462274
- PMCID: PMC2888279
- DOI: 10.1021/bi100068u
Pharmacological characterization of 30 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists, synthetic agonists, and the endogenous agouti-related protein antagonist
Abstract
The melanocortin-4 receptor (MC4R) is a G-protein-coupled receptor (GPCR) that is expressed in the central nervous system and has a role in regulating feeding behavior, obesity, energy homeostasis, male erectile response, and blood pressure. Since the report of the MC4R knockout mouse in 1997, the field has been searching for links between this genetic biomarker and human obesity and type 2 diabetes. More then 80 single nucleotide polymorphisms (SNPs) have been identified from human patients, both obese and nonobese controls. Many significant studies have been performed examining the pharmacological characteristics of these hMC4R SNPs in attempts to identify a molecular defects/insights that might link a genetic factor to the obese phenotype observed in patients possessing these mutations. Our laboratory has previously reported the pharmacological characterization of 40 of these polymorphic hMC4 receptors with multiple endogenous and synthetic ligands. The goal of the current study is to perform a similar comprehensive side-by-side characterization of 30 additional human hMC4R with single nucleotide polymorphisms using multiple endogenous agonists [alpha-, beta-, and gamma(2)-melanocyte stimulating hormones (MSH) and adrenocorticotropin (ACTH)], the antagonist agouti-related protein hAGRP(87-132), and synthetic agonists [NDP-MSH, MTII, and the tetrapeptide Ac-His-dPhe-Arg-Trp-NH(2) (JRH887-9)]. These in vitro data, in some cases, provide a putative molecular link between dysfunctional hMC4R's and human obesity. These 30 hMC4R SNPs include R7H, R18H, R18L, S36Y, P48S, V50M, F51L, E61K, I69T, D90N, S94R, G98R, I121T, A154D, Y157S, W174C, G181D, F202L, A219 V, I226T, G231S, G238D, N240S, C271R, S295P, P299L, E308K, I317V, L325F, and 750DelGA. All but the N240S hMC4R were identified in obese patients. Additionally, we have characterized a double I102T/V103I hMC4R. In addition to the pharmacological characterization, the hMC4R variants were evaluated for cell surface expression by flow cytometry. The F51L, I69T, and A219V hMC4Rs possessed full agonist activity and significantly decreased endogenous agonist ligand potency. At the E61K, D90N, Y157S, and C271R hMC4Rs, all agonist ligands examined were only partially efficacious in generating a maximal signaling response (partial agonists) and possessed significantly decreased endogenous agonist ligand potency. Only the A219V, G238D, and S295P hMC4Rs possessed significantly decreased AGRP(87-132) antagonist potency. These data provide new information for use in GPCR computational development as well as insights into MC4R structure ad function.
Figures

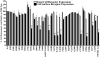
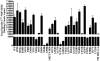
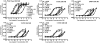
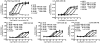

Similar articles
-
Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist.Biochemistry. 2006 Jun 13;45(23):7277-88. doi: 10.1021/bi0600300. Biochemistry. 2006. PMID: 16752916
-
Peptide and small molecules rescue the functional activity and agonist potency of dysfunctional human melanocortin-4 receptor polymorphisms.Biochemistry. 2007 Jul 17;46(28):8273-87. doi: 10.1021/bi7007382. Epub 2007 Jun 23. Biochemistry. 2007. PMID: 17590021
-
Identification of tetrapeptides from a mixture based positional scanning library that can restore nM full agonist function of the L106P, I69T, I102S, A219V, C271Y, and C271R human melanocortin-4 polymorphic receptors (hMC4Rs).J Med Chem. 2014 Jun 12;57(11):4615-28. doi: 10.1021/jm500064t. Epub 2014 May 14. J Med Chem. 2014. PMID: 24517312 Free PMC article.
-
Structural Complexity and Plasticity of Signaling Regulation at the Melanocortin-4 Receptor.Int J Mol Sci. 2020 Aug 10;21(16):5728. doi: 10.3390/ijms21165728. Int J Mol Sci. 2020. PMID: 32785054 Free PMC article. Review.
-
Agouti-related protein: more than a melanocortin-4 receptor antagonist?Peptides. 2005 Oct;26(10):1759-70. doi: 10.1016/j.peptides.2004.11.036. Peptides. 2005. PMID: 15996791 Review.
Cited by
-
Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort.Int J Obes (Lond). 2017 Jan;41(1):13-22. doi: 10.1038/ijo.2016.161. Epub 2016 Sep 22. Int J Obes (Lond). 2017. PMID: 27654141
-
Incorporation of a bioactive reverse-turn heterocycle into a peptide template using solid-phase synthesis to probe melanocortin receptor selectivity and ligand conformations by 2D 1H NMR.J Med Chem. 2011 Mar 10;54(5):1379-90. doi: 10.1021/jm101425m. Epub 2011 Feb 9. J Med Chem. 2011. PMID: 21306168 Free PMC article.
-
Identification of the MC4R start lost mutation in a morbidly obese Brazilian patient.Diabetes Metab Syndr Obes. 2019 Feb 21;12:257-266. doi: 10.2147/DMSO.S189455. eCollection 2019. Diabetes Metab Syndr Obes. 2019. PMID: 30863132 Free PMC article.
-
Effects of glucagon-like peptide-1 analogue treatment in genetic obesity: A case series.Clin Obes. 2021 Dec;11(6):e12481. doi: 10.1111/cob.12481. Epub 2021 Jul 21. Clin Obes. 2021. PMID: 34291582 Free PMC article.
-
Recommended Tool Compounds for the Melanocortin Receptor (MCR) G Protein-Coupled Receptors (GPCRs).ACS Pharmacol Transl Sci. 2024 Aug 26;7(9):2706-2724. doi: 10.1021/acsptsci.4c00129. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296259
References
-
- Lerner AB, McGuire JS. Effect of Alpha- and Beta-Melanocyte Stimulating Hormones on the Skin Colour of Man. Nature. 1961;189:176–179. - PubMed
-
- Bertolini A, Vergoni W, Gessa GL, Ferrari W. Induction of Sexual Excitement by the Action of Adrenocorticotrophic Hormone in Brain. Nature. 1969;221:667–669. - PubMed
-
- Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic Melanotropic Peptide Initiates Erections in Men with Psychogenic Erectile Dysfunction: Double-blind, Placebo Controlled Crossover Study. J Urol. 1998;160:389–393. - PubMed
-
- Lindner E, Scholkens B. ACTH and alpha-MSH: cardiovascular and antiarrhythmic properties. Arch Int Pharmacodyn Ther. 1974;208:19–23. - PubMed
-
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N Engl J Med. 2009;360:44–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

