Rhinorrhea, cough and fatigue in patients taking sitagliptin
- PMID: 20462426
- PMCID: PMC2877018
- DOI: 10.1186/1710-1492-6-8
Rhinorrhea, cough and fatigue in patients taking sitagliptin
Abstract
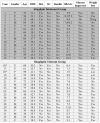
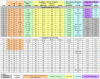
Sitagliptin is a dipeptidyl peptidase-4 (DPP IV, CD26) inhibitor indicated for treatment of Type II diabetes as a second line therapy after metformin. We report fifteen sitagliptin intolerant patients who developed anterior and posterior rhinorrhea, cough, dyspnea, and fatigue. Symptoms typically developed within 1 to 8 weeks of starting, and resolved within 1 week of stopping the drug. Peak expiratory flow rates increased 34% in 8 patients who stopped sitagliptin. Similar changes were found in 4 out of 5 persons who had confirmatory readministration. Chart review identified 17 patients who tolerated sitagliptin and had no symptomatic changes. The sitagliptin intolerant group had higher rates of clinically diagnosed allergic rhinitis (15/15 vs. 6/18; p = 0.00005), Fisher's Exact test) and angiotensin converting enzyme inhibitor - induced cough (6/13 vs. 1/18; p = 0.012). Nasal and inhaled glucocorticoids may control the underlying allergic inflammation and abrogate this new sitagliptin - induced pharmacological syndrome. Potential mucosal and central nervous system mechanisms include disruption of neuropeptides and/or cytokines that rely on DPP IV for activation or inactivation, and T cell dysfunction.
Figures

Similar articles
-
Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin.Diabetes Obes Metab. 2007 Sep;9(5):733-45. doi: 10.1111/j.1463-1326.2007.00744.x. Epub 2007 Jun 26. Diabetes Obes Metab. 2007. PMID: 17593236 Clinical Trial.
-
DPP-4 (CD26) inhibitor sitagliptin exerts anti-inflammatory effects on rat insulinoma (RINm) cells via suppressing NF-κB activation.Endocrine. 2017 Mar;55(3):754-763. doi: 10.1007/s12020-016-1073-8. Epub 2016 Sep 9. Endocrine. 2017. PMID: 27612849
-
Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers.Clin Ther. 2006 Jan;28(1):55-72. doi: 10.1016/j.clinthera.2006.01.015. Clin Ther. 2006. PMID: 16490580 Clinical Trial.
-
Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes.Ann Pharmacother. 2006 Jul-Aug;40(7-8):1336-43. doi: 10.1345/aph.1G665. Ann Pharmacother. 2006. PMID: 16868220 Review.
-
Pharmacokinetic and pharmacodynamic evaluation of sitagliptin plus metformin.Expert Opin Drug Metab Toxicol. 2010 Oct;6(10):1265-76. doi: 10.1517/17425255.2010.513699. Expert Opin Drug Metab Toxicol. 2010. PMID: 20707611 Review.
Cited by
-
Revised Korean Cough Guidelines, 2020: Recommendations and Summary Statements.Tuberc Respir Dis (Seoul). 2021 Oct;84(4):263-273. doi: 10.4046/trd.2021.0038. Epub 2021 May 13. Tuberc Respir Dis (Seoul). 2021. PMID: 33979988 Free PMC article. Review.
-
DPP4 and ACE2 in Diabetes and COVID-19: Therapeutic Targets for Cardiovascular Complications?Front Pharmacol. 2020 Aug 7;11:1161. doi: 10.3389/fphar.2020.01161. eCollection 2020. Front Pharmacol. 2020. PMID: 32848769 Free PMC article. Review.
-
Falsely Accused? Insufficient Evidence to Conclude that Sitagliptin is a Cause of Chronic Cough.Lung. 2020 Apr;198(2):271-273. doi: 10.1007/s00408-020-00329-2. Lung. 2020. PMID: 31980869 No abstract available.
-
SARS-CoV-2 and DPP4 inhibition: Is it time to pray for Janus Bifrons?Diabetes Res Clin Pract. 2020 May;163:108162. doi: 10.1016/j.diabres.2020.108162. Epub 2020 Apr 23. Diabetes Res Clin Pract. 2020. PMID: 32335097 Free PMC article.
-
SARS-CoV-2 and diabetes: A potential therapeutic effect of dipeptidyl peptidase 4 inhibitors in diabetic patients diagnosed with COVID-19.Metabol Open. 2021 Dec;12:100134. doi: 10.1016/j.metop.2021.100134. Epub 2021 Oct 13. Metabol Open. 2021. PMID: 34661092 Free PMC article.
References
-
- Anon. Circular Number 9762802. Merck & Co., Inc. Whitehouse Station, NJ 088893; Januvia (sitagliptin) tablets.
-
- Göke B, Hershon K, Kerr D, Calle Pascual A, Schweizer A, Foley J, Shao Q, Dejager S. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40(12):892–5. doi: 10.1055/s-0028-1082334. - DOI - PubMed
-
- Anon. Circular Number 1256316. Bristol-Meyers Squibb, Princeton, NJ 08543; 2009. Onglyza (saxagliptin) tablets.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

