Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress
- PMID: 20462515
- PMCID: PMC2893230
- DOI: 10.1016/j.cellsig.2010.04.009
Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress
Abstract
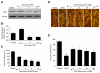
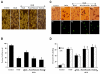
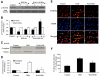
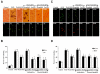
Elucidating the underlying mechanisms that govern microglial activation and survival is essential for the development of new treatment strategies for neurodegenerative disorders, since microglia serve not only as guardian sentries of the nervous system, but also play a significant role in determining neuronal and vascular cell fate. Here we show that endogenous and exogenous Wnt1 in inflammatory microglial cells is necessary for the prevention of apoptotic early membrane phosphatidylserine exposure and later DNA degradation, since blockade of Wnt1 signaling abrogates cell survival during oxidative stress. Wnt1 prevents apoptotic demise through the post-translational phosphorylation and maintenance of FoxO3a in the cytoplasm to inhibit an apoptotic cascade that relies upon the loss of mitochondrial membrane permeability, cytochrome c release, Bad phosphorylation, and activation of caspase 3 and caspase 1 as demonstrated by complimentary gene knockdown studies of FoxO3a. Furthermore, subcellular trafficking and gene knockdown studies of NF-kappaB p65 illustrate that microglial cell survival determined by Wnt1 during oxidative stress requires NF-kappaB p65. Our work highlights Wnt1 and the control of novel downstream transcriptional pathways as critical components for the oversight of nervous system microglial cells.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis.Curr Neurovasc Res. 2009 Nov;6(4):223-38. doi: 10.2174/156720209789630302. Curr Neurovasc Res. 2009. PMID: 19807657 Free PMC article.
-
Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation.Curr Neurovasc Res. 2010 May;7(2):95-112. doi: 10.2174/156720210791184899. Curr Neurovasc Res. 2010. PMID: 20370652 Free PMC article.
-
Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia.Curr Neurovasc Res. 2011 Nov;8(4):270-85. doi: 10.2174/156720211798120990. Curr Neurovasc Res. 2011. PMID: 22023617 Free PMC article.
-
Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system.Histol Histopathol. 2004 Apr;19(2):495-504. doi: 10.14670/HH-19.495. Histol Histopathol. 2004. PMID: 15024710 Free PMC article. Review.
-
Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease.Prog Neurobiol. 2005 Feb;75(3):207-46. doi: 10.1016/j.pneurobio.2005.02.004. Epub 2005 Apr 26. Prog Neurobiol. 2005. PMID: 15882775 Review.
Cited by
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
-
Innovative therapeutic strategies for cardiovascular disease.EXCLI J. 2023 Jul 26;22:690-715. doi: 10.17179/excli2023-6306. eCollection 2023. EXCLI J. 2023. PMID: 37593239 Free PMC article. Review.
-
Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss.Curr Neurovasc Res. 2017;14(4):415-420. doi: 10.2174/1567202614666171116102911. Curr Neurovasc Res. 2017. PMID: 29149835 Free PMC article. Review.
-
Changes of Foxo3a in PBMCs and its associations with stress hyperglycemia in acute obstructive suppurative cholangitis patients.Oncotarget. 2017 Aug 7;8(44):76783-76796. doi: 10.18632/oncotarget.20011. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100348 Free PMC article.
-
Cannabidiol and the Canonical WNT/β-Catenin Pathway in Glaucoma.Int J Mol Sci. 2021 Apr 6;22(7):3798. doi: 10.3390/ijms22073798. Int J Mol Sci. 2021. PMID: 33917605 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

