In utero tobacco exposure epigenetically modifies placental CYP1A1 expression
- PMID: 20462615
- PMCID: PMC2921565
- DOI: 10.1016/j.metabol.2010.01.013
In utero tobacco exposure epigenetically modifies placental CYP1A1 expression
Abstract
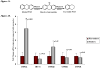
The metabolic pathways used by higher-eukaryotic organisms to deal with potentially carcinogenic xenobiotic compounds from tobacco smoke have been well characterized. Carcinogenic compounds such as polycyclic aromatic hydrocarbons are metabolized sequentially in 2 phases: in phase I, CYP1A1 catalyzes conversion into harmful hydrophilic DNA adducts, whereas in phase II, GSTT1 enables excretion via conjugation into polar electrophiles. In an effort to understand susceptibility to in utero tobacco exposure, we previously characterized known metabolic functional polymorphisms and demonstrated that although deletion of fetal GSTT1 significantly modified birth weight in smokers, no polymorphism fully accounted for fetal growth restriction. Because smoking up-regulates CYP1A1 expression, we hypothesized that nonallelic (epigenetic) dysregulation of placental CYP1A1 expression via alterations in DNA methylation (meCpG) may further modify fetal growth. In the present article, we compared placental expression of multiple CYP family members among gravidae and observed significantly increased CYP1A1 expression among smokers relative to controls (4.4-fold, P < .05). To fully characterize CYP1A1 meCpG status, bisulfite modification and sequencing of the entire proximal 1-kilobase promoter (containing 59 CpG sites) were performed. CpG sites immediately proximal to the 5′-xenobiotic response element transcription factor binding element were significantly hypomethylated among smokers (55.6% vs 45.9% meCpG, P = .027), a finding that uniquely correlated with placental gene expression (r = 0.737, P = .007). Thus, in utero tobacco exposure significantly increases placental CYP1A1 expression in association with differential methylation at a critical xenobiotic response element.
Conflict of interest statement
The authors have no conflicts of interest nor financial disclosures.
Figures

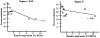
References
-
- Smits KM, Benhamou S, Garte S, Weijenberg MP, Alamanos Y, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, et al. Association of metabolic gene polymorphisms with tobacco consumption in healthy controls. Int J Cancer. 2004;110:266–270. - PubMed
-
- Larsen JE, Colosimo ML, Yang IA, Bowman R, Zimmerman PV, Fong KM. CYP1A1 Ile462Val and MPO G-463A interact to increase risk of adenocarcinoma but not squamous cell carcinoma of the lung. Carcinogenesis. 2006;27:525–532. - PubMed
-
- Hou L, Chatterjee N, Huang WY, Baccarelli A, Yadavalli S, Yeager M, Bresalier RS, Chanock SJ, Caporaso NE, Ji BT, et al. CYP1A1 Val462 and NQO1 Ser187 polymorphisms, cigarette use, and risk for colorectal adenoma. Carcinogenesis. 2005;26:1122–1128. - PubMed
-
- Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, Lacoursiere DY. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am J Obstet Gynecol. 2008;198:66, e61–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

