Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction
- PMID: 20462867
- PMCID: PMC2920809
- DOI: 10.1093/cvr/cvq141
Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction
Abstract
Aims: Coronary artery occlusion resulting in ischaemia/reperfusion (I/R) injury is a major cause of mortality in the western world. Circulating natural IgM has been shown to play a significant role in reperfusion injury, leading to the notion of a pathogenic response against self by the innate immune system. A specific self-antigen (non-muscle myosin heavy chain II) was recently identified as the major target of pathogenic natural IgM. Therefore, we hypothesized that a synthetic peptide mimetope (N2) or monoclonal antibodies directed against the self-antigen would prevent specific IgM binding to the self-antigen and reduce reperfusion injury in the heart.
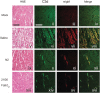
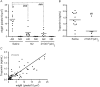
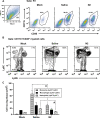
Methods and results: We find that treatment with N2 peptide reduces infarct size by 47% and serum cardiac troponin-I levels by 69% following 1 h ischaemia and 24 h reperfusion. N2 peptide or an anti-N2 F(ab')(2) (21G6) is also effective at preventing IgM and complement deposition. Additionally, N2 peptide treatment significantly reduces monocyte and neutrophil infiltration at 24 h and collagen deposition at 5 days. Finally, we show that human IgM (hIgM) also includes specificity for the highly conserved self-antigen and that myocardial injury in antibody-deficient mice reconstituted with hIgM is blocked by treatment with N2 peptide or 21G6 F(ab')(2).
Conclusion: The findings in this study identify potential therapeutics [i.e. N2 peptide or 21G6 F(ab')(2)] that prevent specific IgM binding to ischaemic antigens in the heart, resulting in a significant reduction in cardiac I/R injury.
Figures



Comment in
-
Nothing but natural: targeting natural IgM in ischaemia/reperfusion injury.Cardiovasc Res. 2010 Sep 1;87(4):589-90. doi: 10.1093/cvr/cvq209. Epub 2010 Jun 23. Cardiovasc Res. 2010. PMID: 20573731 No abstract available.
References
-
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Contran Pathologic Basis of Disease. Professional Edition. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010.
-
- Wu B, Ootani A, Iwakiri R, Fujise T, Tsunada S, Toda S, et al. Ischemic preconditioning attenuates ischemia-reperfusion-induced mucosal apoptosis by inhibiting the mitochondria-dependent pathway in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G580–G587. doi:10.1152/ajpgi.00335.2003. - DOI - PubMed
-
- Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA. 1997;94:679–683. doi:10.1073/pnas.94.2.679. - DOI - PMC - PubMed
-
- Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi:10.1056/NEJMoa071142. - DOI - PubMed
-
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi:10.1161/01.CIR.0000101682.24138.36. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

