Free edges in epithelial cell sheets stimulate epidermal growth factor receptor signaling
- PMID: 20462956
- PMCID: PMC2893982
- DOI: 10.1091/mbc.e09-12-1026
Free edges in epithelial cell sheets stimulate epidermal growth factor receptor signaling
Abstract
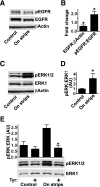
The ability of epithelia to migrate and cover wounds is essential to maintaining their functions as physical barriers. Wounding induces many cues that may affect the transition to motility, including the immediate mechanical perturbation, release of material from broken cells, new interactions with adjacent extracellular matrix, and breakdown of physical separation of ligands from their receptors. Depending on the exact nature of wounds, some cues may be present only transiently or insignificantly. In many epithelia, activation of the epidermal growth factor receptor (EGFR) is a central event in induction of motility, and we find that its continuous activation is required for progression of healing of wounds in sheets of corneal epithelial cells. Here, we examine the hypothesis that edges, which are universally and continuously present in wounds, are a cue. Using a novel culture model we find that their presence is sufficient to cause activation of the EGFR and increased motility of cells in the absence of other cues. Edges that are bordered by agarose do not induce activation of the EGFR, indicating that activation is not due to loss of any specific type of cell-cell interaction but rather due to loss of physical constraints.
Figures






References
-
- Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. - PubMed
-
- Bindschadler M., McGrath J. L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 2007;120:876–884. - PubMed
-
- Bjorklund M., Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta. 2005;1755:37–69. - PubMed
-
- Block E. R., Matela A. R., SundarRaj N., Iszkula E. R., Klarlund J. K. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J. Biol. Chem. 2004;279:24307–24312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

