Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion
- PMID: 20462967
- PMCID: PMC2928528
- DOI: 10.1152/ajprenal.00120.2010
Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion
Abstract
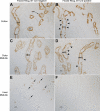
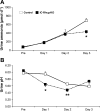
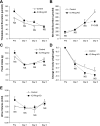
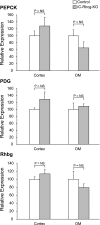
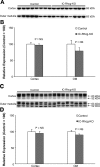
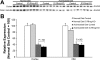
Rh C glycoprotein (Rhcg) is an NH(3)-specific transporter expressed in both intercalated cells (IC) and principal cells (PC) in the renal collecting duct. Recent studies show that deletion of Rhcg from both intercalated and principal cells inhibits both basal and acidosis-stimulated renal ammonia excretion. The purpose of the current studies was to better understand the specific role of Rhcg expression in intercalated cells in basal and metabolic acidosis-stimulated renal ammonia excretion. We generated mice with intercalated cell-specific Rhcg deletion (IC-Rhcg-KO) using Cre-loxP techniques; control (C) mice were floxed Rhcg but Cre negative. Under basal conditions, IC-Rhcg-KO and C mice excreted urine with similar ammonia content and pH. Mice were then acid loaded by adding HCl to their diet. Ammonia excretion after acid loading increased similarly in IC-Rhcg-KO and C mice during the first 2 days of acid loading but on day 3 was significantly less in IC-Rhcg-KO than in C mice. During the first 2 days of acid loading, urine was significantly more acidic in IC-Rhcg-KO mice than in C mice; there was no difference on day 3. In IC-Rhcg-KO mice, acid loading increased principal cell Rhcg expression in both the cortex and outer medulla as well as expression of another ammonia transporter, Rh glycoprotein B (Rhbg), in principal cells in the outer medulla. We conclude that 1) Rhcg expression in intercalated cells is necessary for the normal renal response to metabolic acidosis; 2) principal cell Rhcg contributes to both basal and acidosis-stimulated ammonia excretion; and 3) adaptations in Rhbg expression occur in response to acid-loading.
Figures

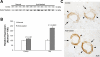

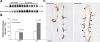
References
-
- Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 - PubMed
-
- Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 - PubMed
-
- Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003 - PubMed
-
- Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

