Group II introns: mobile ribozymes that invade DNA
- PMID: 20463000
- PMCID: PMC3140690
- DOI: 10.1101/cshperspect.a003616
Group II introns: mobile ribozymes that invade DNA
Abstract
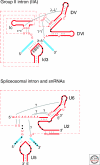
Group II introns are mobile ribozymes that self-splice from precursor RNAs to yield excised intron lariat RNAs, which then invade new genomic DNA sites by reverse splicing. The introns encode a reverse transcriptase that stabilizes the catalytically active RNA structure for forward and reverse splicing, and afterwards converts the integrated intron RNA back into DNA. The characteristics of group II introns suggest that they or their close relatives were evolutionary ancestors of spliceosomal introns, the spliceosome, and retrotransposons in eukaryotes. Further, their ribozyme-based DNA integration mechanism enabled the development of group II introns into gene targeting vectors ("targetrons"), which have the unique feature of readily programmable DNA target specificity.
Figures


References
-
- Aizawa Y, Xiang Q, Lambowitz AM, Pyle AM 2003. The pathway for DNA recognition and RNA integration by a group II intron retrotransposon. Mol Cell 11: 795–805 - PubMed
-
- Barkan A 2004. Intron splicing in plant organelles. In Molecular biology and biotechnology of plant organelles (eds Daniell H, Chase C), pp. 281–308 Kluwer Academic Publishers, Dordrecht
-
- Barkan A 2009. Genome-wide analysis of RNA-protein interactions in plants. Methods Mol Biol 553: 13–37 - PubMed
-
- Belfort M, Derbyshire V, Parker MM, Cousineau B, Lambowitz AM 2002. Mobile introns: Pathways and proteins. In Mobile DNA II (eds Craig NL, Craigie R, Gellert M, Lambowitz AM), pp. 761–783 ASM Press, Washington D.C
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
