Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva
- PMID: 20463014
- PMCID: PMC2903413
- DOI: 10.1074/jbc.M109.094557
Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva
Abstract
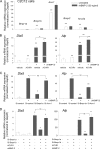
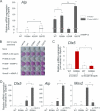
Fibrodysplasia ossificans progressiva (FOP), a rare genetic and catastrophic disorder characterized by progressive heterotopic ossification, is caused by a point mutation, c.617G>A; p.R206H, in the activin A receptor type 1 (ACVR1) gene, one of the bone morphogenetic protein type I receptors (BMPR-Is). Although altered BMP signaling has been suggested to explain the pathogenesis, the molecular consequences of this mutation are still elusive. Here we studied the impact of ACVR1 R206H mutation on BMP signaling and its downstream signaling cascades in murine myogenic C2C12 cells and HEK 293 cells. We found that ACVR1 was the most abundant of the BMPR-Is expressed in mesenchymal cells but its contribution to osteogenic BMP signal transduction was minor. The R206H mutant caused weak activation of the BMP signaling pathway, unlike the Q207D mutant, a strong and constitutively active form. The R206H mutant showed a decreased binding affinity for FKBP1A/FKBP12, a known safeguard molecule against the leakage of transforming growth factor (TGF)-beta or BMP signaling. The decreased binding affinity of FKBP1A to the mutant R206H ACVR1 resulted in leaky activation of the BMP signal, and moreover, it decreased steady-state R206H ACVR1 protein levels. Interestingly, while WT ACVR1 and FKBP1A were broadly distributed in plasma membrane and cytoplasm without BMP-2 stimulation and then localized in plasma membrane on BMP-2 stimulation, R206H ACVR1 and FKBP1A were mainly distributed in plasma membrane regardless of BMP-2 stimulation. The impaired binding to FKBP1A and an altered subcellular distribution by R206H ACVR1 mutation may result in mild activation of osteogenic BMP-signaling in extraskeletal sites such as muscle, which eventually lead to delayed and progressive ectopic bone formation in FOP patients.
Figures




References
-
- Connor J. M., Evans D. A. (1982) J. Bone Joint. Surg. Br. 64, 76–83 - PubMed
-
- Shafritz A. B., Shore E. M., Gannon F. H., Zasloff M. A., Taub R., Muenke M., Kaplan F. S. (1996) N. Engl. J. Med. 335, 555–561 - PubMed
-
- Cohen R. B., Hahn G. V., Tabas J. A., Peeper J., Levitz C. L., Sando A., Sando N., Zasloff M., Kaplan F. S. (1993) J. Bone Joint. Surg. Am. 75, 215–219 - PubMed
-
- Kitterman J. A., Kantanie S., Rocke D. M., Kaplan F. S. (2005) Pediatrics 116, e654–661 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

