Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase
- PMID: 20463019
- PMCID: PMC2903410
- DOI: 10.1074/jbc.M110.111559
Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase
Abstract
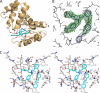
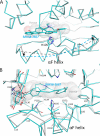
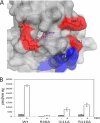
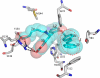
Heme is a vital molecule for all life forms with heme being capable of assisting in catalysis, binding ligands, and undergoing redox changes. Heme-related dysfunction can lead to cardiovascular diseases with the oxidation of the heme of soluble guanylyl cyclase (sGC) critically implicated in some of these cardiovascular diseases. sGC, the main nitric oxide (NO) receptor, stimulates second messenger cGMP production, whereas reactive oxygen species are known to scavenge NO and oxidize/inactivate the heme leading to sGC degradation. This vulnerability of NO-heme signaling to oxidative stress led to the discovery of an NO-independent activator of sGC, cinaciguat (BAY 58-2667), which is a candidate drug in clinical trials to treat acute decompensated heart failure. Here, we present crystallographic and mutagenesis data that reveal the mode of action of BAY 58-2667. The 2.3-A resolution structure of BAY 58-2667 bound to a heme NO and oxygen binding domain (H-NOX) from Nostoc homologous to that of sGC reveals that the trifurcated BAY 58-2667 molecule has displaced the heme and acts as a heme mimetic. Carboxylate groups of BAY 58-2667 make interactions similar to the heme-propionate groups, whereas its hydrophobic phenyl ring linker folds up within the heme cavity in a planar-like fashion. BAY 58-2667 binding causes a rotation of the alphaF helix away from the heme pocket, as this helix is normally held in place via the inhibitory His(105)-heme covalent bond. The structure provides insights into how BAY 58-2667 binds and activates sGC to rescue heme-NO dysfunction in cardiovascular diseases.
Figures


Similar articles
-
Insight into the rescue of oxidized soluble guanylate cyclase by the activator cinaciguat.Chembiochem. 2012 May 7;13(7):977-81. doi: 10.1002/cbic.201100809. Epub 2012 Mar 30. Chembiochem. 2012. PMID: 22474005 Free PMC article.
-
Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes.Biochemistry. 2013 May 21;52(20):3601-8. doi: 10.1021/bi301657w. Epub 2013 May 7. Biochemistry. 2013. PMID: 23614626 Free PMC article.
-
Insights into soluble guanylyl cyclase activation derived from improved heme-mimetics.J Med Chem. 2013 Nov 14;56(21):8948-8952. doi: 10.1021/jm400539d. Epub 2013 Oct 24. J Med Chem. 2013. PMID: 24090476 Free PMC article.
-
Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease.Cardiovasc Drug Rev. 2007 Spring;25(1):30-45. doi: 10.1111/j.1527-3466.2007.00003.x. Cardiovasc Drug Rev. 2007. PMID: 17445086 Review.
-
How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase.Antioxid Redox Signal. 2012 Nov 1;17(9):1246-63. doi: 10.1089/ars.2012.4564. Epub 2012 Apr 10. Antioxid Redox Signal. 2012. PMID: 22356101 Free PMC article. Review.
Cited by
-
Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme.Proc Natl Acad Sci U S A. 2012 Aug 7;109(32):12998-3003. doi: 10.1073/pnas.1205854109. Epub 2012 Jul 25. Proc Natl Acad Sci U S A. 2012. PMID: 22837396 Free PMC article.
-
Nitric oxide activation of guanylate cyclase pushes the α1 signaling helix and the β1 heme-binding domain closer to the substrate-binding site.J Biol Chem. 2014 Jan 3;289(1):476-84. doi: 10.1074/jbc.M113.504472. Epub 2013 Nov 12. J Biol Chem. 2014. PMID: 24220034 Free PMC article.
-
Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content.J Biol Chem. 2014 May 30;289(22):15259-71. doi: 10.1074/jbc.M114.559393. Epub 2014 Apr 14. J Biol Chem. 2014. PMID: 24733395 Free PMC article.
-
Nitric Oxide System and Bronchial Epithelium: More Than a Barrier.Front Physiol. 2021 Jun 30;12:687381. doi: 10.3389/fphys.2021.687381. eCollection 2021. Front Physiol. 2021. PMID: 34276407 Free PMC article. Review.
-
Insight into the rescue of oxidized soluble guanylate cyclase by the activator cinaciguat.Chembiochem. 2012 May 7;13(7):977-81. doi: 10.1002/cbic.201100809. Epub 2012 Mar 30. Chembiochem. 2012. PMID: 22474005 Free PMC article.
References
-
- Faller M., Matsunaga M., Yin S., Loo J. A., Guo F. (2007) Nat. Struct. Mol. Biol. 14, 23–29 - PubMed
-
- Krishnamurthy P., Xie T., Schuetz J. D. (2007) Pharmacol. Ther. 114, 345–358 - PubMed
-
- Gilles-Gonzalez M. A., Gonzalez G. (2005) J. Inorg. Biochem. 99, 1–22 - PubMed
-
- Bryan N. S., Bian K., Murad F. (2009) Front Biosci. 14, 1–18 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

