Molecular and functional basis for the scaffolding role of the p50/dynamitin subunit of the microtubule-associated dynactin complex
- PMID: 20463029
- PMCID: PMC2906295
- DOI: 10.1074/jbc.M110.100602
Molecular and functional basis for the scaffolding role of the p50/dynamitin subunit of the microtubule-associated dynactin complex
Abstract
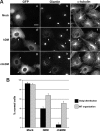
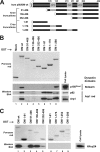
p50/dynamitin (DM) is a major subunit of the microtubule-associated dynactin complex that is required for stabilization and attachment of its two distinct structural domains, namely the Arp1 rod and the shoulder/sidearm. Here, we define the determinants of p50/DM required for self-oligomerization of the protein and for interactions with other subunits of the dynactin complex. Whereas the N-terminal 1-91-amino acid region of the protein is required and sufficient for binding to the Arp1 rod, additional determinants contained within the first half of the protein are required for optimal recruitment of the p150(Glued) subunit of the shoulder/sidearm. Overexpression experiments confirmed that the N-terminal 1-91-amino acid region of p50/DM is critical for dynactin functionality, because this fragment acts as a dominant negative to inhibit both dynein-dependent and -independent functions of the complex. Furthermore, the first two predicted coiled-coil motifs of p50/DM contain determinants that mediate self-association of the protein. Interestingly, p50/DM self-association does not contribute to p50/DM-induced disruption of the dynactin complex, but most likely participates in the stabilization of the complex.
Figures

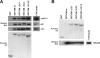


Similar articles
-
Dynactin integrity depends upon direct binding of dynamitin to Arp1.Mol Biol Cell. 2014 Jul 15;25(14):2171-80. doi: 10.1091/mbc.E14-03-0842. Epub 2014 May 14. Mol Biol Cell. 2014. PMID: 24829381 Free PMC article.
-
Mechanism of dynamitin-mediated disruption of dynactin.J Biol Chem. 2007 Jul 6;282(27):19355-64. doi: 10.1074/jbc.M700003200. Epub 2007 Apr 22. J Biol Chem. 2007. PMID: 17449914
-
Dynamitin mutagenesis reveals protein-protein interactions important for dynactin structure.Traffic. 2008 Apr;9(4):481-91. doi: 10.1111/j.1600-0854.2008.00702.x. Epub 2008 Jan 7. Traffic. 2008. PMID: 18182012 Free PMC article.
-
Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the arp1 minifilament pointed end.J Cell Biol. 1999 Oct 18;147(2):307-20. doi: 10.1083/jcb.147.2.307. J Cell Biol. 1999. PMID: 10525537 Free PMC article.
-
Dynactin.Annu Rev Cell Dev Biol. 2004;20:759-79. doi: 10.1146/annurev.cellbio.20.012103.094623. Annu Rev Cell Dev Biol. 2004. PMID: 15473859 Review.
Cited by
-
Dynactin integrity depends upon direct binding of dynamitin to Arp1.Mol Biol Cell. 2014 Jul 15;25(14):2171-80. doi: 10.1091/mbc.E14-03-0842. Epub 2014 May 14. Mol Biol Cell. 2014. PMID: 24829381 Free PMC article.
-
A SILAC-based proteomics elicits the molecular interactome of alisertib (MLN8237) in human erythroleukemia K562 cells.Am J Transl Res. 2015 Nov 15;7(11):2442-61. eCollection 2015. Am J Transl Res. 2015. PMID: 26807190 Free PMC article.
-
The Chlamydia effector Dre1 binds dynactin to reposition host organelles during infection.Cell Rep. 2025 Apr 22;44(4):115509. doi: 10.1016/j.celrep.2025.115509. Epub 2025 Apr 4. Cell Rep. 2025. PMID: 40186871 Free PMC article.
-
Three pairs of surrogate redox partners comparison for Class I cytochrome P450 enzyme activity reconstitution.Commun Biol. 2022 Aug 6;5(1):791. doi: 10.1038/s42003-022-03764-4. Commun Biol. 2022. PMID: 35933448 Free PMC article.
-
The Salmonella effector SteA contributes to the control of membrane dynamics of Salmonella-containing vacuoles.Infect Immun. 2014 Jul;82(7):2923-34. doi: 10.1128/IAI.01385-13. Epub 2014 Apr 28. Infect Immun. 2014. PMID: 24778114 Free PMC article.
References
-
- Muhua L., Karpova T. S., Cooper J. A. (1994) Cell 78, 669–679 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

