Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors
- PMID: 20463034
- PMCID: PMC2875842
- DOI: 10.1242/dev.052126
Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors
Abstract
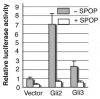
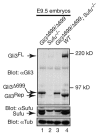
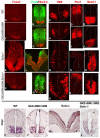
Gli2 and Gli3 are primary transcriptional regulators that mediate hedgehog (Hh) signaling. Mechanisms that stabilize and destabilize Gli2 and Gli3 are essential for the proteins to promptly respond to Hh signaling or to be inactivated following the activation. In this study, we show that loss of suppressor of fused (Sufu; an inhibitory effector for Gli proteins) results in destabilization of Gli2 and Gli3 full-length activators but not of their C-terminally processed repressors, whereas overexpression of Sufu stabilizes them. By contrast, RNAi knockdown of Spop (a substrate-binding adaptor for the cullin3-based ubiquitin E3 ligase) in Sufu mutant mouse embryonic fibroblasts (MEFs) can restore the levels of Gli2 and Gli3 full-length proteins, but not those of their repressors, whereas introducing Sufu into the MEFs stabilizes Gli2 and Gli3 full-length proteins and rescues Gli3 processing. Consistent with these findings, forced Spop expression promotes Gli2 and Gli3 degradation and Gli3 processing. The functions of Sufu and Spop oppose each other through their competitive binding to the N- and C-terminal regions of Gli3 or the C-terminal region of Gli2. More importantly, the Gli3 repressor expressed by a Gli3 mutant allele (Gli3(Delta699)) can mostly rescue the ventralized neural tube phenotypes of Sufu mutant embryos, indicating that the Gli3 repressor can function independently of Sufu. Our study provides a new insight into the regulation of Gli2 and Gli3 stability and processing by Sufu and Spop, and reveals the unexpected Sufu-independent Gli3 repressor function.
Figures





Similar articles
-
Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved.Genes Dev. 2009 Aug 15;23(16):1910-28. doi: 10.1101/gad.1794109. Genes Dev. 2009. PMID: 19684112 Free PMC article.
-
Spop regulates Gli3 activity and Shh signaling in dorsoventral patterning of the mouse spinal cord.Dev Biol. 2017 Dec 1;432(1):72-85. doi: 10.1016/j.ydbio.2017.04.002. Epub 2017 Apr 12. Dev Biol. 2017. PMID: 28412462 Free PMC article.
-
Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation.Development. 2000 Apr;127(8):1593-605. doi: 10.1242/dev.127.8.1593. Development. 2000. PMID: 10725236
-
The sonic hedgehog-patched-gli pathway in human development and disease.Am J Hum Genet. 2000 Nov;67(5):1047-54. doi: 10.1016/S0002-9297(07)62934-6. Epub 2000 Sep 21. Am J Hum Genet. 2000. PMID: 11001584 Free PMC article. Review. No abstract available.
-
Gli proteins and the control of spinal-cord patterning.EMBO Rep. 2003 Aug;4(8):761-5. doi: 10.1038/sj.embor.embor896. EMBO Rep. 2003. PMID: 12897799 Free PMC article. Review.
Cited by
-
T-box3 is a ciliary protein and regulates stability of the Gli3 transcription factor to control digit number.Elife. 2016 Apr 5;5:e07897. doi: 10.7554/eLife.07897. Elife. 2016. PMID: 27046536 Free PMC article.
-
What are those cilia doing in the neural tube?Cilia. 2012 Oct 1;1(1):19. doi: 10.1186/2046-2530-1-19. Cilia. 2012. PMID: 23351466 Free PMC article.
-
A Comparison of Ci/Gli Activity as Regulated by Sufu in Drosophila and Mammalian Hedgehog Response.PLoS One. 2015 Aug 13;10(8):e0135804. doi: 10.1371/journal.pone.0135804. eCollection 2015. PLoS One. 2015. PMID: 26271100 Free PMC article.
-
Regulation of Hedgehog signaling Offers A Novel Perspective for Bone Homeostasis Disorder Treatment.Int J Mol Sci. 2019 Aug 16;20(16):3981. doi: 10.3390/ijms20163981. Int J Mol Sci. 2019. PMID: 31426273 Free PMC article. Review.
-
Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube.Development. 2011 Nov;138(22):4921-30. doi: 10.1242/dev.070805. Epub 2011 Oct 17. Development. 2011. PMID: 22007132 Free PMC article.
References
-
- Aza-Blanc P., Ramirez-Weber F. A., Laget M. P., Schwartz C., Kornberg T. B. (1997). Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89, 1043-1053 - PubMed
-
- Bai C. B., Stephen D., Joyner A. L. (2004). All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103-115 - PubMed
-
- Barnfield P. C., Zhang X., Thanabalasingham V., Yoshida M., Hui C. C. (2005). Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation 73, 397-405 - PubMed
-
- Bose J., Grotewold L., Ruther U. (2002). Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum. Mol. Genet. 11, 1129-1135 - PubMed
-
- Briscoe J., Ericson J. (2001). Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 11, 43-49 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

