Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation
- PMID: 20463052
- PMCID: PMC2903934
- DOI: 10.1210/en.2010-0016
Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation
Erratum in
- Endocrinology. 2011 Jan;152(1):336
Abstract
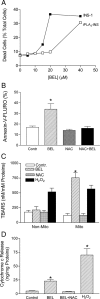
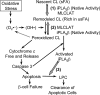
Mitochondrial production of reactive oxygen species and oxidation of cardiolipin are key events in initiating apoptosis. We reported that group VIA Ca(2+)-independent phospholipase A(2) (iPLA(2)beta) localizes in and protects beta-cell mitochondria from oxidative damage during staurosporine-induced apoptosis. Here, we used iPLA(2)beta-null (iPLA(2)beta(-/-)) mice to investigate the role of iPLA(2)beta in the repair of mitochondrial membranes. We show that islets isolated from iPLA(2)beta(-/-) mice are more sensitive to staurosporine-induced apoptosis than those from wild-type littermates and that 2 wk of daily ip administration of staurosporine to iPLA(2)beta(-/-) mice impairs both the animals' glucose tolerance and glucose-stimulated insulin secretion by their pancreatic islets. Moreover, the iPLA(2)beta inhibitor bromoenol lactone caused mitochondrial membrane peroxidation and cytochrome c release, and these effects were reversed by N-acetyl cysteine. The mitochondrial antioxidant N-t-butyl hydroxylamine blocked staurosporine-induced cytochrome c release and caspase-3 activation in iPLA(2)beta(-/-) islets. Furthermore, the collapse of mitochondrial membrane potential in INS-1 insulinoma cells caused by high glucose and fatty acid levels was attenuated by overexpressing iPLA(2)beta. Interestingly, iPLA(2)beta was expressed only at low levels in islet beta-cells from obesity- and diabetes-prone db/db mice. These findings support the hypothesis that iPLA(2)beta is important in repairing oxidized mitochondrial membrane components (e.g. cardiolipin) and that this prevents cytochrome c release in response to stimuli that otherwise induce apoptosis. The low iPLA(2)beta expression level in db/db mouse beta-cells may render them vulnerable to injury by reactive oxygen species.
Figures





Similar articles
-
Group VIA phospholipase A2 mitigates palmitate-induced β-cell mitochondrial injury and apoptosis.J Biol Chem. 2014 May 16;289(20):14194-210. doi: 10.1074/jbc.M114.561910. Epub 2014 Mar 19. J Biol Chem. 2014. PMID: 24648512 Free PMC article.
-
Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2beta in pancreatic beta-cells and in iPLA2beta-null mice.Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E217-29. doi: 10.1152/ajpendo.00474.2007. Epub 2007 Sep 25. Am J Physiol Endocrinol Metab. 2008. PMID: 17895289 Free PMC article.
-
Group VIA PLA2 (iPLA2β) is activated upstream of p38 mitogen-activated protein kinase (MAPK) in pancreatic islet β-cell signaling.J Biol Chem. 2012 Feb 17;287(8):5528-41. doi: 10.1074/jbc.M111.285114. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194610 Free PMC article.
-
The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β -cell failure.Curr Diabetes Rev. 2012 Jan;8(1):69-75. doi: 10.2174/157339912798829232. Curr Diabetes Rev. 2012. PMID: 22414059 Free PMC article. Review.
-
Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus.Exp Diabetes Res. 2012;2012:703538. doi: 10.1155/2012/703538. Epub 2011 Nov 9. Exp Diabetes Res. 2012. PMID: 22110477 Free PMC article. Review.
Cited by
-
Mitochondrial membrane lipids in the regulation of bioenergetic flux.Cell Metab. 2024 Sep 3;36(9):1963-1978. doi: 10.1016/j.cmet.2024.07.024. Epub 2024 Aug 22. Cell Metab. 2024. PMID: 39178855 Free PMC article. Review.
-
Mitochondrial dysfunction and reduced prostaglandin synthesis in skeletal muscle of Group VIB Ca2+-independent phospholipase A2gamma-deficient mice.J Lipid Res. 2010 Oct;51(10):3003-15. doi: 10.1194/jlr.M008060. Epub 2010 Jul 12. J Lipid Res. 2010. PMID: 20625036 Free PMC article.
-
Genetic ablation of PLA2G6 in mice leads to cerebellar atrophy characterized by Purkinje cell loss and glial cell activation.PLoS One. 2011;6(10):e26991. doi: 10.1371/journal.pone.0026991. Epub 2011 Oct 28. PLoS One. 2011. PMID: 22046428 Free PMC article.
-
High expression of α-synuclein in damaged mitochondria with PLA2G6 dysfunction.Acta Neuropathol Commun. 2016 Mar 30;4:27. doi: 10.1186/s40478-016-0298-3. Acta Neuropathol Commun. 2016. PMID: 27030050 Free PMC article.
-
Neuroprotective efficacy of N-t-butylhydroxylamine (NtBHA) in transient focal ischemia in rats.Toxicol Res. 2022 Apr 14;38(4):479-486. doi: 10.1007/s43188-022-00131-7. eCollection 2022 Oct. Toxicol Res. 2022. PMID: 36277357 Free PMC article.
References
-
- Mathis D, Vence L, Benoist C 2001 β-Cell death during progression to diabetes. Nature 414:792–798 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 - PubMed
-
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ 1993 Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241–251 - PubMed
-
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG 2005 Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1:223–232 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

