Oligomerization of Ebola virus VP40 is essential for particle morphogenesis and regulation of viral transcription
- PMID: 20463076
- PMCID: PMC2898221
- DOI: 10.1128/JVI.00737-10
Oligomerization of Ebola virus VP40 is essential for particle morphogenesis and regulation of viral transcription
Abstract
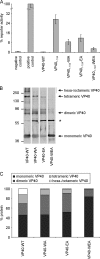
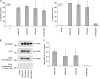
The morphogenesis and budding of virus particles represent an important stage in the life cycle of viruses. For Ebola virus, this process is driven by its major matrix protein, VP40. Like the matrix proteins of many other nonsegmented, negative-strand RNA viruses, VP40 has been demonstrated to oligomerize and to occur in at least two distinct oligomeric states: hexamers and octamers, which are composed of antiparallel dimers. While it has been shown that VP40 oligomers are essential for the viral life cycle, their function is completely unknown. Here we have identified two amino acids essential for oligomerization of VP40, the mutation of which blocked virus-like particle production. Consistent with this observation, oligomerization-deficient VP40 also showed impaired intracellular transport to budding sites and reduced binding to cellular membranes. However, other biological functions, such as the interaction of VP40 with the nucleoprotein, NP, remained undisturbed. Furthermore, both wild-type VP40 and oligomerization-deficient VP40 were found to negatively regulate viral genome replication, a novel function of VP40, which we have recently reported. Interestingly, while wild-type VP40 was also able to negatively regulate viral genome transcription, oligomerization-deficient VP40 was no longer able to fulfill this function, indicating that regulation of viral replication and transcription by VP40 are mechanistically distinct processes. These data indicate that VP40 oligomerization not only is a prerequisite for intracellular transport of VP40 and efficient membrane binding, and as a consequence virion morphogenesis, but also plays a critical role in the regulation of viral transcription by VP40.
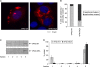
Figures

References
-
- Becker, S., C. Rinne, U. Hofsass, H. D. Klenk, and E. Muhlberger. 1998. Interactions of Marburg virus nucleocapsid proteins. Virology 249:406-417. - PubMed
-
- Boehmann, Y., S. Enterlein, A. Randolf, and E. Muhlberger. 2005. A reconstituted replication and transcription system for Ebola virus Reston and comparison with Ebola virus Zaire. Virology 332:406-417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

