OrfA downregulates feline immunodeficiency virus primary receptor CD134 on the host cell surface and is important in viral infection
- PMID: 20463078
- PMCID: PMC2898258
- DOI: 10.1128/JVI.00434-10
OrfA downregulates feline immunodeficiency virus primary receptor CD134 on the host cell surface and is important in viral infection
Abstract
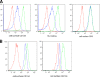
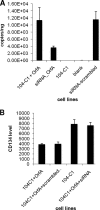
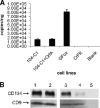
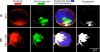
Feline immunodeficiency virus (FIV) OrfA is an accessory protein that is critical for productive viral replication and infection in T cells. Here, we show that OrfA acts to markedly reduce cell surface expression of the FIV primary binding receptor. Downregulation does not occur at the transcriptional or translational level in that the amounts of CD134 mRNA and protein in total cell lysates are not altered between parental 104-C1 T cells and the same cell line stably expressing OrfA (104-C1-OrfA). Analysis by confocal microscopy revealed significant accumulation of CD134 in the Golgi apparatus of 104-C1 cells expressing OrfA. OrfA does not cause a generalized disruption of membrane trafficking in that surface expression of CD9 is unaffected by OrfA overexpression. Consistent with the above observations, OrfA-negative FIV-34TF10 productively infects CrFK (CD134-negative) and 104-C1-OrfA (CD134 downregulated by OrfA) cells but fails to productively infect either 104-C1 (CD134-positive) cells or GFox (CrFK cells overexpressing CD134) cells. FIV-34TF10 in which the OrfA reading frame is open (OrfArep) productively infects CrFK, GFox, 104-C1, and 104-C1-OrfA cells. We hypothesize that reduced surface expression of the receptor, a hallmark of retrovirus infections, may facilitate an increase in virus release from the infected cell by minimizing receptor interactions with budding virus particles.
Figures

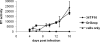
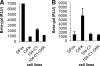
Similar articles
-
Feline immunodeficiency virus OrfA alters gene expression of splicing factors and proteasome-ubiquitination proteins.Virology. 2008 Feb 20;371(2):394-404. doi: 10.1016/j.virol.2007.09.039. Epub 2007 Oct 25. Virology. 2008. PMID: 17963812 Free PMC article.
-
CD134 and CXCR4 expression corresponds to feline immunodeficiency virus infection of lymphocytes, macrophages and dendritic cells.J Gen Virol. 2008 Jan;89(Pt 1):277-287. doi: 10.1099/vir.0.83161-0. J Gen Virol. 2008. PMID: 18089752
-
Expression of CD134 and CXCR4 mRNA in term placentas from FIV-infected and control cats.Vet Immunol Immunopathol. 2008 May 15;123(1-2):90-6. doi: 10.1016/j.vetimm.2008.01.014. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18295905 Free PMC article.
-
The virus-receptor interaction in the replication of feline immunodeficiency virus (FIV).Curr Opin Virol. 2013 Dec;3(6):670-5. doi: 10.1016/j.coviro.2013.08.003. Epub 2013 Aug 28. Curr Opin Virol. 2013. PMID: 23992667 Free PMC article. Review.
-
[Feline immunodeficiency virus tropism].Uirusu. 2007 Jun;57(1):75-82. doi: 10.2222/jsv.57.75. Uirusu. 2007. PMID: 18040157 Review. Japanese.
Cited by
-
Accessory genes confer a high replication rate to virulent feline immunodeficiency virus.J Virol. 2013 Jul;87(14):7940-51. doi: 10.1128/JVI.00752-13. Epub 2013 May 8. J Virol. 2013. PMID: 23658451 Free PMC article.
-
Feline immunodeficiency virus envelope glycoproteins antagonize tetherin through a distinctive mechanism that requires virion incorporation.J Virol. 2014 Mar;88(6):3255-72. doi: 10.1128/JVI.03814-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390322 Free PMC article.
-
Modulation of ADAM17 Levels by Pestiviruses Is Species-Specific.Viruses. 2024 Oct 2;16(10):1564. doi: 10.3390/v16101564. Viruses. 2024. PMID: 39459898 Free PMC article.
-
Properties and Functions of Feline Immunodeficiency Virus Gag Domains in Virion Assembly and Budding.Viruses. 2018 May 16;10(5):261. doi: 10.3390/v10050261. Viruses. 2018. PMID: 29772651 Free PMC article. Review.
-
The molecular biology of feline immunodeficiency virus (FIV).Viruses. 2011 Nov;3(11):2192-213. doi: 10.3390/v3112192. Epub 2011 Nov 9. Viruses. 2011. PMID: 22163340 Free PMC article. Review.
References
-
- de Parseval, A., U. Chatterji, G. Morris, P. Sun, A. J. Olson, and J. H. Elder. 2005. Structural mapping of CD134 residues critical for interaction with feline immunodeficiency virus. Nat. Struct. Mol. Biol. 12:60-66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

