Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells
- PMID: 20463083
- PMCID: PMC2898267
- DOI: 10.1128/JVI.00576-10
Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells
Abstract
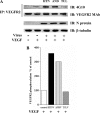
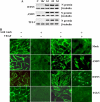
Hantaviruses infect endothelial cells and cause 2 vascular permeability-based diseases. Pathogenic hantaviruses enhance the permeability of endothelial cells in response to vascular endothelial growth factor (VEGF). However, the mechanism by which hantaviruses hyperpermeabilize endothelial cells has not been defined. The paracellular permeability of endothelial cells is uniquely determined by the homophilic assembly of vascular endothelial cadherin (VE-cadherin) within adherens junctions, which is regulated by VEGF receptor-2 (VEGFR2) responses. Here, we investigated VEGFR2 phosphorylation and the internalization of VE-cadherin within endothelial cells infected by pathogenic Andes virus (ANDV) and Hantaan virus (HTNV) and nonpathogenic Tula virus (TULV) hantaviruses. We found that VEGF addition to ANDV- and HTNV-infected endothelial cells results in the hyperphosphorylation of VEGFR2, while TULV infection failed to increase VEGFR2 phosphorylation. Concomitant with the VEGFR2 hyperphosphorylation, VE-cadherin was internalized to intracellular vesicles within ANDV- or HTNV-, but not TULV-, infected endothelial cells. Addition of angiopoietin-1 (Ang-1) or sphingosine-1-phosphate (S1P) to ANDV- or HTNV-infected cells blocked VE-cadherin internalization in response to VEGF. These findings are consistent with the ability of Ang-1 and S1P to inhibit hantavirus-induced endothelial cell permeability. Our results suggest that pathogenic hantaviruses disrupt fluid barrier properties of endothelial cell adherens junctions by enhancing VEGFR2-VE-cadherin pathway responses which increase paracellular permeability. These results provide a pathway-specific mechanism for the enhanced permeability of hantavirus-infected endothelial cells and suggest that stabilizing VE-cadherin within adherens junctions is a primary target for regulating endothelial cell permeability during pathogenic hantavirus infection.
Figures


References
-
- Aird, W. C. 2004. Endothelium as an organ system. Crit. Care Med. 32:S271-S279. - PubMed
-
- Berger, M. M., C. Hesse, C. Dehnert, H. Siedler, P. Kleinbongard, H. J. Bardenheuer, M. Kelm, P. Bartsch, and W. E. Haefeli. 2005. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 172:763-767. - PubMed
-
- Borges, E., Y. Jan, and E. Ruoslahti. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867-39873. - PubMed
-
- Bustamante, E. A., H. Levy, and S. Q. Simpson. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 112:1133-1136. - PubMed
-
- Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

