Variation of herbivore-induced volatile terpenes among Arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03
- PMID: 20463089
- PMCID: PMC2899926
- DOI: 10.1104/pp.110.154864
Variation of herbivore-induced volatile terpenes among Arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03
Abstract
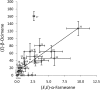
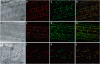
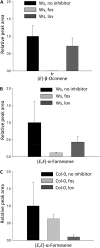
When attacked by insects, plants release mixtures of volatile compounds that are beneficial for direct or indirect defense. Natural variation of volatile emissions frequently occurs between and within plant species, but knowledge of the underlying molecular mechanisms is limited. We investigated intraspecific differences of volatile emissions induced from rosette leaves of 27 accessions of Arabidopsis (Arabidopsis thaliana) upon treatment with coronalon, a jasmonate mimic eliciting responses similar to those caused by insect feeding. Quantitative variation was found for the emission of the monoterpene (E)-beta-ocimene, the sesquiterpene (E,E)-alpha-farnesene, the irregular homoterpene 4,8,12-trimethyltridecatetra-1,3,7,11-ene, and the benzenoid compound methyl salicylate. Differences in the relative emissions of (E)-beta-ocimene and (E,E)-alpha-farnesene from accession Wassilewskija (Ws), a high-(E)-beta-ocimene emitter, and accession Columbia (Col-0), a trace-(E)-beta-ocimene emitter, were attributed to allelic variation of two closely related, tandem-duplicated terpene synthase genes, TPS02 and TPS03. The Ws genome contains a functional allele of TPS02 but not of TPS03, while the opposite is the case for Col-0. Recombinant proteins of the functional Ws TPS02 and Col-0 TPS03 genes both showed (E)-beta-ocimene and (E,E)-alpha-farnesene synthase activities. However, differential subcellular compartmentalization of the two enzymes in plastids and the cytosol was found to be responsible for the ecotype-specific differences in (E)-beta-ocimene/(E,E)-alpha-farnesene emission. Expression of the functional TPS02 and TPS03 alleles is induced in leaves by elicitor and insect treatment and occurs constitutively in floral tissues. Our studies show that both pseudogenization in the TPS family and subcellular segregation of functional TPS enzymes control the variation and plasticity of induced volatile emissions in wild plant species.
Figures


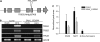
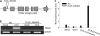


References
-
- Aharoni A, Jongsma MA, Bouwmeester HJ. (2005) Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci 10: 594–602 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

