On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl- cotransport activity
- PMID: 20463172
- PMCID: PMC2944316
- DOI: 10.1152/ajpcell.00074.2010
On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl- cotransport activity
Abstract
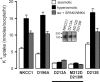
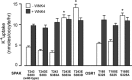
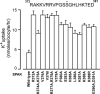
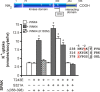
Threonines targeted by Ste20-related proline-alanine-rich kinase (SPAK) for phosphorylation have been identified in Na+-K+-2Cl(-) cotransporter type 1 (NKCC1), NKCC2, and Na+-Cl(-) cotransporter (NCC). However, what constitutes the substrate recognition of the kinase is still unknown. Using site-directed mutagenesis and functional measurement of NKCC1 activity in Xenopus laevis oocytes, we determined that SPAK recognizes two threonine residues separated by four amino acids. Addition or removal of a single residue abrogated SPAK activation of NKCC1. Although both threonines are followed by hydrophobic residues, in vivo experiments have determined that SPAK activation of the cotransporter only requires a hydrophobic residue after the first threonine. Interestingly, downstream of the second threonine residue, we have identified a conserved aspartic acid residue which is critical for NKCC1 function. Mouse SPAK activity requires phosphorylation of two specific residues by WNK [with no lysine (K)] kinases: a threonine (T243) in the catalytic domain and a serine (S383) in the regulatory domain. We found that mutating the threonine residue into a glutamic acid (T243E) combined with mutation of the serine into an aspartic acid (S383D) rendered SPAK constitutively active. Surprisingly, alanine substitution of S383 or mutation of residues surrounding this residue also resulted in a constitutively active kinase. Interestingly, deletion of amino acids 356-398 identified another serine residue in the catalytic domain (S321) as another putative target of WNK phosphorylation. We found that WNK4 is capable of stimulating the deletion mutant when S321 is present, but not when S321 is mutated into an alanine.
Figures



References
-
- Ching YP, Leong VY, Wong CM, Kung HF. Identification of an autoinhibitory domain of p21-activated protein kinase 5. J Biol Chem 278: 33621–33624, 2003 - PubMed
-
- Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem 271: 21049–21053, 1996 - PubMed
-
- Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem 45: 773–778, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

