Gamma band unit activity and population responses in the pedunculopontine nucleus
- PMID: 20463196
- PMCID: PMC2904222
- DOI: 10.1152/jn.00242.2010
Gamma band unit activity and population responses in the pedunculopontine nucleus
Abstract
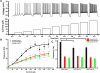
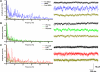
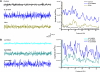
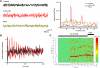
The pedunculopontine nucleus (PPN) is involved in the activated states of waking and paradoxical sleep, forming part of the reticular activating system (RAS). The studies described tested the hypothesis that single unit and/or population responses of PPN neurons are capable of generating gamma band frequency activity. Whole cell patch clamp recordings (immersion chamber) and population responses (interface chamber) were conducted on 9- to 20-day-old rat brain stem slices. Regardless of cell type (I, II, or III) or type of response to the nonselective cholinergic receptor agonist carbachol (excitation, inhibition, biphasic), almost all PPN neurons fired at gamma band frequency, but no higher, when subjected to depolarizing steps (50 +/- 2 Hz, mean +/- SE). Nonaccommodating neurons fired at 18-100 Hz throughout depolarizing steps, while most accommodating neurons exhibited gamma band frequency of action potentials followed by gamma band membrane oscillations. These oscillations were blocked by the sodium channel blocker tetrodotoxin (TTX), suggesting that at least some are mediated by sodium currents. Population responses in the PPN showed that carbachol induced peaks of activation in the theta and gamma range, while glutamatergic receptor agonists induced overall increases in activity at theta and gamma frequencies, although in differing patterns. Gamma band activity appears to be a part of the intrinsic membrane properties of PPN neurons, and the population as a whole generates different patterns of gamma band activity under the influence of specific transmitters. Given sufficient excitation, the PPN may impart gamma band activation on its targets.
Figures


References
-
- Azouz R, Gray CM, Nowak LG, McCormick DA. Phsyiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex 7: 534–545, 1997 - PubMed
-
- Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience 143: 739–755, 2006 - PMC - PubMed
-
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol 87: 1790–1798, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

