A relative position code for saccades in dorsal premotor cortex
- PMID: 20463216
- PMCID: PMC2887302
- DOI: 10.1523/JNEUROSCI.1625-09.2010
A relative position code for saccades in dorsal premotor cortex
Abstract
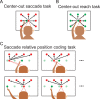
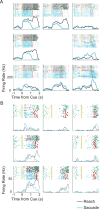
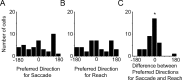
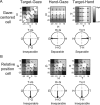
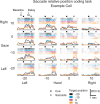
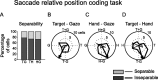
Spatial computations underlying the coordination of the hand and eye present formidable geometric challenges. One way for the nervous system to simplify these computations is to directly encode the relative position of the hand and the center of gaze. Neurons in the dorsal premotor cortex (PMd), which is critical for the guidance of arm-reaching movements, encode the relative position of the hand, gaze, and goal of reaching movements. This suggests that PMd can coordinate reaching movements with eye movements. Here, we examine saccade-related signals in PMd to determine whether they also point to a role for PMd in coordinating visual-motor behavior. We first compared the activity of a population of PMd neurons with a population of parietal reach region (PRR) neurons. During center-out reaching and saccade tasks, PMd neurons responded more strongly before saccades than PRR neurons, and PMd contained a larger proportion of exclusively saccade-tuned cells than PRR. During a saccade relative position-coding task, PMd neurons encoded saccade targets in a relative position code that depended on the relative position of gaze, the hand, and the goal of a saccadic eye movement. This relative position code for saccades is similar to the way that PMd neurons encode reach targets. We propose that eye movement and eye position signals in PMd do not drive eye movements, but rather provide spatial information that links the control of eye and arm movements to support coordinated visual-motor behavior.
Figures




Similar articles
-
Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning.Neuron. 2006 Jul 6;51(1):125-34. doi: 10.1016/j.neuron.2006.05.025. Neuron. 2006. PMID: 16815337 Free PMC article.
-
Saccade-related activity in the parietal reach region.J Neurophysiol. 2000 Feb;83(2):1099-102. doi: 10.1152/jn.2000.83.2.1099. J Neurophysiol. 2000. PMID: 10669521
-
Implementation of spatial transformation rules for goal-directed reaching via gain modulation in monkey parietal and premotor cortex.J Neurosci. 2009 Jul 29;29(30):9490-9. doi: 10.1523/JNEUROSCI.1095-09.2009. J Neurosci. 2009. PMID: 19641112 Free PMC article.
-
Parieto-frontal coding of reaching: an integrated framework.Exp Brain Res. 1999 Dec;129(3):325-46. doi: 10.1007/s002210050902. Exp Brain Res. 1999. PMID: 10591906 Review.
-
Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones.Brain. 2002 Feb;125(Pt 2):225-37. doi: 10.1093/brain/awf034. Brain. 2002. PMID: 11844724 Review.
Cited by
-
Specialization of reach function in human posterior parietal cortex.Exp Brain Res. 2012 Aug;221(1):1-18. doi: 10.1007/s00221-012-3158-9. Epub 2012 Jul 10. Exp Brain Res. 2012. PMID: 22777102 Review.
-
Cognition in Sensorimotor Control: Interfacing With the Posterior Parietal Cortex.Front Neurosci. 2019 Feb 27;13:140. doi: 10.3389/fnins.2019.00140. eCollection 2019. Front Neurosci. 2019. PMID: 30872993 Free PMC article.
-
Keeping the world at hand: rapid visuomotor processing for hand-object interactions.Exp Brain Res. 2012 Jun;219(4):421-8. doi: 10.1007/s00221-012-3089-5. Epub 2012 Apr 17. Exp Brain Res. 2012. PMID: 22526949 Review.
-
Lesions of cortical area LIP affect reach onset only when the reach is accompanied by a saccade, revealing an active eye-hand coordination circuit.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2371-6. doi: 10.1073/pnas.1220508110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341626 Free PMC article.
-
Mapping Eye, Arm, and Reward Information in Frontal Motor Cortices Using Electrocorticography in Nonhuman Primates.J Neurosci. 2025 Mar 19;45(12):e1536242025. doi: 10.1523/JNEUROSCI.1536-24.2025. J Neurosci. 2025. PMID: 39890467
References
-
- Batschelet E. Circular statistics in biology. New York: Academic; 1981.
-
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex. 2001;11:528–544. - PubMed
-
- Battaglia-Mayer A, Mascaro M, Caminiti R. Temporal evolution and strength of neural activity in parietal cortex during eye and hand movements. Cereb Cortex. 2007;17:1350–1363. - PubMed
-
- Blohm G, Crawford JD. Computations for geometrically accurate visually guided reaching in 3-D space. J Vis. 2007;7:4.1–4.22. - PubMed
-
- Blohm G, Keith GP, Crawford JD. Decoding the cortical transformations for visually guided reaching in 3D space. Cereb Cortex. 2009;19:1372–1393. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
