Tissue plasminogen activator alters intracellular sequestration of zinc through interaction with the transporter ZIP4
- PMID: 20463217
- PMCID: PMC2872103
- DOI: 10.1523/JNEUROSCI.6250-09.2010
Tissue plasminogen activator alters intracellular sequestration of zinc through interaction with the transporter ZIP4
Abstract
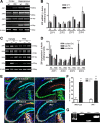
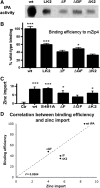
Glutamatergic neurons contain free zinc packaged into neurotransmitter-loaded synaptic vesicles. Upon neuronal activation, the vesicular contents are released into the synaptic space, whereby the zinc modulates activity of postsynaptic neurons though interactions with receptors, transporters and exchangers. However, high extracellular concentrations of zinc trigger seizures and are neurotoxic if substantial amounts of zinc reenter the cells via ion channels and accumulate in the cytoplasm. Tissue plasminogen activator (tPA), a secreted serine protease, is also proepileptic and excitotoxic. However, tPA counters zinc toxicity by promoting zinc import back into the neurons in a sequestered form that is nontoxic. Here, we identify the zinc influx transporter, ZIP4, as the pathway through which tPA mediates the zinc uptake. We show that ZIP4 is upregulated after excitotoxin stimulation of the mouse, male and female, hippocampus. ZIP4 physically interacts with tPA, correlating with an increased intracellular zinc influx and lysosomal sequestration. Changes in prosurvival signals support the idea that this sequestration results in neuroprotection. These experiments identify a mechanism via which neurons use tPA to efficiently neutralize the toxic effects of excessive concentrations of free zinc.
Figures





Similar articles
-
Modulation of zinc toxicity by tissue plasminogen activator.Mol Cell Neurosci. 2004 Jan;25(1):162-71. doi: 10.1016/j.mcn.2003.10.007. Mol Cell Neurosci. 2004. PMID: 14962749
-
Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury.J Neurosci. 2003 Apr 15;23(8):3234-42. doi: 10.1523/JNEUROSCI.23-08-03234.2003. J Neurosci. 2003. PMID: 12716930 Free PMC article.
-
Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus.J Neurosci. 2002 Mar 15;22(6):2125-34. doi: 10.1523/JNEUROSCI.22-06-02125.2002. J Neurosci. 2002. PMID: 11896152 Free PMC article.
-
Tissue plasminogen activator as a modulator of neuronal survival and function.Biochem Soc Trans. 2002 Apr;30(2):222-5. Biochem Soc Trans. 2002. PMID: 12023855 Review.
-
Clinical implications of the involvement of tPA in neuronal cell death.J Mol Med (Berl). 1997 May;75(5):341-7. doi: 10.1007/s001090050119. J Mol Med (Berl). 1997. PMID: 9181475 Review.
Cited by
-
Cytosolic zinc release and clearance in hippocampal neurons exposed to glutamate--the role of pH and sodium.J Neurochem. 2011 Apr;117(2):231-43. doi: 10.1111/j.1471-4159.2011.07194.x. Epub 2011 Mar 1. J Neurochem. 2011. PMID: 21255017 Free PMC article.
-
Zinc-triggered induction of tissue plasminogen activator and plasminogen in endothelial cells and pericytes.Exp Neurobiol. 2013 Dec;22(4):315-21. doi: 10.5607/en.2013.22.4.315. Epub 2013 Dec 31. Exp Neurobiol. 2013. PMID: 24465147 Free PMC article.
-
Modulation of neuronal signal transduction and memory formation by synaptic zinc.Front Behav Neurosci. 2011 Nov 9;5:68. doi: 10.3389/fnbeh.2011.00068. eCollection 2011. Front Behav Neurosci. 2011. PMID: 22084630 Free PMC article.
-
Environmental toxicants and male reproductive function.Spermatogenesis. 2011 Jan;1(1):2-13. doi: 10.4161/spmg.1.1.13971. Spermatogenesis. 2011. PMID: 21866273 Free PMC article.
-
Cognitive decline due to excess synaptic Zn(2+) signaling in the hippocampus.Front Aging Neurosci. 2014 Feb 27;6:26. doi: 10.3389/fnagi.2014.00026. eCollection 2014. Front Aging Neurosci. 2014. PMID: 24578691 Free PMC article. Review.
References
-
- Andrade-Gordon P, Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986;25:4033–4040. - PubMed
-
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. - PubMed
-
- Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am J Physiol Heart Circ Physiol. 2000;279:H1346–H1354. - PubMed
-
- Coulter D. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered “epileptic” gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia. 2000;41(Suppl 6):S96–S99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
