Identification of Erbin interlinking MuSK and ErbB2 and its impact on acetylcholine receptor aggregation at the neuromuscular junction
- PMID: 20463225
- PMCID: PMC6632580
- DOI: 10.1523/JNEUROSCI.5778-09.2010
Identification of Erbin interlinking MuSK and ErbB2 and its impact on acetylcholine receptor aggregation at the neuromuscular junction
Abstract
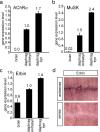
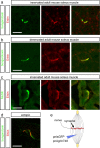
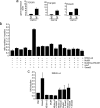
Erbin, a binding partner of ErbB2, was identified as the first member of the LAP family of proteins. Erbin was shown at postsynaptic membranes of the neuromuscular junction (NMJ) or in cultured C2C12 myotubes (1) to be concentrated, (2) to regulate the Ras-Raf-Mek pathway, and (3) to inhibit TGF-beta signaling. In the CNS, Erbin interacts with PSD-95. Furthermore, agrin-MuSK signaling initiates formation of AChR aggregates at the postsynaptic membrane. In search of proteins interacting with MuSK, we identified Erbin as a MuSK binding protein. We verified the interaction of MuSK with Erbin, or both concomitantly with ErbB2 by coimmunoprecipitation, and we mapped the interacting epitopes between Erbin and MuSK. We demonstrated elevated mRNA levels of Erbin at synaptic nuclei and colocalized Erbin and MuSK at postsynaptic membranes. We identified several Erbin isoforms at the NMJ, all of which contained the MuSK binding domain. By knocking down Erbin, we observed agrin-dependent AChR aggregates on murine primary skeletal myotubes and C2C12 cells, and in the absence of agrin, microclusters, both of significantly lower density. Complementary, AChR-epsilon-reporter expression was reduced in myotubes overexpressing Erbin. We show that myotubes also express other LAP protein family members, namely Scribble and Lano, and that both affect physical dimensions of agrin-dependent AChR aggregates and density of microclusters formed in the absence of agrin. Moreover, MuSK-Erbin-ErbB2 signaling influences TGF-beta signaling. Our data define the requirement of Erbin on the cross talk between agrin and neuregulin signaling pathways at the NMJ.
Figures







Similar articles
-
Tyrosine phosphatases such as SHP-2 act in a balance with Src-family kinases in stabilization of postsynaptic clusters of acetylcholine receptors.BMC Neurosci. 2007 Jul 2;8:46. doi: 10.1186/1471-2202-8-46. BMC Neurosci. 2007. PMID: 17605785 Free PMC article.
-
The Agrin/MuSK signaling pathway is spatially segregated from the neuregulin/ErbB receptor signaling pathway at the neuromuscular junction.J Neurosci. 2000 Dec 1;20(23):8762-70. doi: 10.1523/JNEUROSCI.20-23-08762.2000. J Neurosci. 2000. PMID: 11102484 Free PMC article.
-
Tyrosine phosphatase regulation of MuSK-dependent acetylcholine receptor clustering.Mol Cell Neurosci. 2005 Mar;28(3):403-16. doi: 10.1016/j.mcn.2004.10.005. Mol Cell Neurosci. 2005. PMID: 15737732
-
Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation.Cell Mol Life Sci. 2013 Sep;70(17):3077-88. doi: 10.1007/s00018-012-1209-9. Epub 2012 Nov 22. Cell Mol Life Sci. 2013. PMID: 23178848 Free PMC article. Review.
-
MuSk function during health and disease.Neurosci Lett. 2020 Jan 18;716:134676. doi: 10.1016/j.neulet.2019.134676. Epub 2019 Dec 4. Neurosci Lett. 2020. PMID: 31811897 Review.
Cited by
-
Adenosine Receptors in Developing and Adult Mouse Neuromuscular Junctions and Functional Links With Other Metabotropic Receptor Pathways.Front Pharmacol. 2018 Apr 24;9:397. doi: 10.3389/fphar.2018.00397. eCollection 2018. Front Pharmacol. 2018. PMID: 29740322 Free PMC article.
-
The Upregulation of Leucine-Rich Repeat Containing 1 Expression Activates Hepatic Stellate Cells and Promotes Liver Fibrosis by Stabilizing Phosphorylated Smad2/3.Int J Mol Sci. 2024 Feb 27;25(5):2735. doi: 10.3390/ijms25052735. Int J Mol Sci. 2024. PMID: 38473980 Free PMC article.
-
Erbin interacts with TARP γ-2 for surface expression of AMPA receptors in cortical interneurons.Nat Neurosci. 2013 Mar;16(3):290-9. doi: 10.1038/nn.3320. Epub 2013 Jan 27. Nat Neurosci. 2013. PMID: 23354328
-
Loss of Protein Kinase Csnk2b/CK2β at Neuromuscular Junctions Affects Morphology and Dynamics of Aggregated Nicotinic Acetylcholine Receptors, Neuromuscular Transmission, and Synaptic Gene Expression.Cells. 2019 Aug 20;8(8):940. doi: 10.3390/cells8080940. Cells. 2019. PMID: 31434353 Free PMC article.
-
Myasthenia Gravis: From the Viewpoint of Pathogenicity Focusing on Acetylcholine Receptor Clustering, Trans-Synaptic Homeostasis and Synaptic Stability.Front Mol Neurosci. 2020 May 28;13:86. doi: 10.3389/fnmol.2020.00086. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32547365 Free PMC article.
References
-
- Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. - PubMed
-
- Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adélaïde J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2:407–414. - PubMed
-
- Cheusova T, Khan MA, Enz R, Hashemolhosseini S. Identification of developmentally regulated expression of MuSK in astrocytes of the rodent retina. J Neurochem. 2006a;99:450–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
