IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area
- PMID: 20463231
- PMCID: PMC2881312
- DOI: 10.1523/JNEUROSCI.4453-09.2010
IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area
Abstract
Synaptic plasticity in the mesolimbic dopamine (DA) system is critically involved in reward-based conditioning and the development of drug addiction. Ca2+ signals triggered by postsynaptic action potentials (APs) drive the induction of synaptic plasticity in the CNS. However, it is not clear how AP-evoked Ca2+ signals and the resulting synaptic plasticity are altered during in vivo exposure to drugs of abuse. We have recently described long-term potentiation (LTP) of NMDA receptor (NMDAR)-mediated transmission onto DA neurons that is induced in a manner dependent on bursts of APs. LTP induction requires amplification of burst-evoked Ca2+ signals by preceding activation of metabotropic glutamate receptors (mGluRs) generating inositol 1,4,5-trisphosphate (IP3). In this study, using brain slices prepared from male rats, we show that repeated in vivo exposure to the psychostimulant amphetamine (5 mg/kg, i.p., 3-7 d) upregulates mGluR-dependent facilitation of burst-evoked Ca2+ signals in DA neurons of the ventral tegmental area (VTA). Protein kinase A (PKA)-induced sensitization of IP3 receptors mediates this upregulation of mGluR action. As a consequence, NMDAR-mediated transmission becomes more susceptible to LTP induction after repeated amphetamine exposure. We have also found that the magnitude of amphetamine-conditioned place preference (CPP) in behaving rats correlates with the magnitude of mGluR-dependent Ca2+ signal facilitation measured in VTA slices prepared from these rats. Furthermore, the development of amphetamine CPP is significantly attenuated by intra-VTA infusion of the PKA inhibitor H89. We propose that enhancement of mGluR-dependent NMDAR plasticity in the VTA may promote the learning of environmental stimuli repeatedly associated with amphetamine experience.
Figures
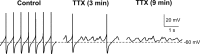








References
-
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. - PubMed
-
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. - PubMed
-
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
