Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity
- PMID: 20463235
- PMCID: PMC2994257
- DOI: 10.1523/JNEUROSCI.4997-09.2010
Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity
Abstract
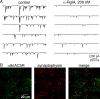
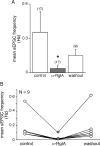
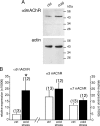
An increase in circulating adrenal catecholamine levels constitutes one of the mechanisms whereby organisms cope with stress. Accordingly, stimulus-secretion coupling within the stressed adrenal medullary tissue undergoes persistent remodeling. In particular, cholinergic synaptic neurotransmission between splanchnic nerve terminals and chromaffin cells is upregulated in stressed rats. Since synaptic transmission is mainly supported by activation of postsynaptic neuronal acetylcholine nicotinic receptors (nAChRs), we focused our study on the role of alpha9-containing nAChRs, which have been recently described in chromaffin cells. Taking advantage of their specific blockade by the alpha-conotoxin RgIA (alpha-RgIA), we unveil novel functional roles for these receptors in the stimulus-secretion coupling of the medulla. First, we show that in rat acute adrenal slices, alpha9-containing nAChRs codistribute with synaptophysin and significantly contribute to EPSCs. Second, we show that these receptors are involved in the tonic inhibitory control exerted by cholinergic activity on gap junctional coupling between chromaffin cells, as evidenced by an increased Lucifer yellow diffusion within the medulla in alpha-RgIA-treated slices. Third, we unexpectedly found that alpha9-containing nAChRs dominantly (>70%) contribute to acetylcholine-induced current in cold-stressed rats, whereas alpha3 nAChRs are the main contributing channels in unstressed animals. Consistently, expression levels of alpha9 nAChR transcript and protein are overexpressed in cold-stressed rats. As a functional relevance, we propose that upregulation of alpha9-containing nAChR channels and ensuing dominant contribution in cholinergic signaling may be one of the mechanisms whereby adrenal medullary tissue appropriately adapts to increased splanchnic nerve electrical discharges occurring in stressful situations.
Figures






Similar articles
-
Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells.J Neurosci. 2003 May 1;23(9):3669-78. doi: 10.1523/JNEUROSCI.23-09-03669.2003. J Neurosci. 2003. PMID: 12736338 Free PMC article.
-
Nicotinic acetylcholine receptors of adrenal chromaffin cells.Acta Physiol (Oxf). 2008 Feb;192(2):203-12. doi: 10.1111/j.1748-1716.2007.01804.x. Epub 2007 Nov 15. Acta Physiol (Oxf). 2008. PMID: 18005395 Review.
-
Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats.J Neurosci. 2008 Jun 25;28(26):6616-26. doi: 10.1523/JNEUROSCI.5597-07.2008. J Neurosci. 2008. PMID: 18579734 Free PMC article.
-
Functional chromaffin cell plasticity in response to stress: focus on nicotinic, gap junction, and voltage-gated Ca2+ channels.J Mol Neurosci. 2012 Oct;48(2):368-86. doi: 10.1007/s12031-012-9707-7. Epub 2012 Jan 18. J Mol Neurosci. 2012. PMID: 22252244 Free PMC article. Review.
-
Overexpression of P2X3 and P2X7 Receptors and TRPV1 Channels in Adrenomedullary Chromaffin Cells in a Rat Model of Neuropathic Pain.Int J Mol Sci. 2019 Jan 3;20(1):155. doi: 10.3390/ijms20010155. Int J Mol Sci. 2019. PMID: 30609840 Free PMC article.
Cited by
-
Corticofugal and Brainstem Functions Associated With Medial Olivocochlear Cholinergic Transmission.Front Neurosci. 2022 Apr 27;16:866161. doi: 10.3389/fnins.2022.866161. eCollection 2022. Front Neurosci. 2022. PMID: 35573302 Free PMC article. Review.
-
Human nicotinic receptors in chromaffin cells: characterization and pharmacology.Pflugers Arch. 2018 Jan;470(1):21-27. doi: 10.1007/s00424-017-2073-0. Epub 2017 Oct 20. Pflugers Arch. 2018. PMID: 29058146 Free PMC article. Review.
-
Gap junction communication between chromaffin cells: the hidden face of adrenal stimulus-secretion coupling.Pflugers Arch. 2018 Jan;470(1):89-96. doi: 10.1007/s00424-017-2032-9. Epub 2017 Jul 22. Pflugers Arch. 2018. PMID: 28735418 Review.
-
The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain.Int J Mol Sci. 2020 Nov 6;21(21):8325. doi: 10.3390/ijms21218325. Int J Mol Sci. 2020. PMID: 33171955 Free PMC article.
-
Brain Regulation of Cardiac Function during Hypoglycemia.Metabolites. 2023 Oct 18;13(10):1089. doi: 10.3390/metabo13101089. Metabolites. 2023. PMID: 37887414 Free PMC article. Review.
References
-
- Baruchin A, Weisberg EP, Miner LL, Ennis D, Nisenbaum LK, Naylor E, Stricker EM, Zigmond MJ, Kaplan BB. Effects of cold exposure on rat adrenal tyrosine hydroxylase: an analysis of RNA, protein, enzyme activity, and cofactor levels. J Neurochem. 1990;54:1769–1775. - PubMed
-
- Campos-Caro A, Smillie FI, Domínguez del Toro E, Rovira JC, Vicente-Agulló F, Chapuli J, Juíz JM, Sala S, Sala F, Ballesta JJ, Criado M. Neuronal nicotinic acetylcholine receptors on bovine chromaffin cells: cloning, expression, and genomic organization of receptor subunits. J Neurochem. 1997;68:488–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
